
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:ced Stoichiometry: Mast
x
+
f1.app.edmentum.com/assessments-delivery/ua/mt/launch/49001627/45422837/aHR0cHM6Ly9mMS5hcHAUZWRtZW50dW0uY29tL2xlYXJuZXItdWkvc2Vjb25kYXJ
dents Chemistry I-Smith -...
1 v Next >
Advanced Stoichiometry: Mastery Test
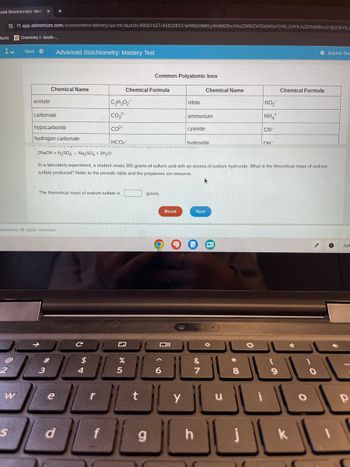
acetate
Chemical Name
carbonate
hypocarbonite
hydrogen carbonate
Common Polyatomic lons
Chemical Formula
Chemical Name
Chemical Formula
C2H3O2
nitrite
NO₂
CO2-
ammonium
NH4+
CO2-
cyanide
CN-
HCO
hvdroxide.
он-
Submit Tes
2NaOH+H2SO4 Na2SO4 + 2H2O.
In a laboratory experiment, a student mixes 355 grams of sulfuric acid with an excess of sodium hydroxide. What is the theoretical mass of sodium
sulfate produced? Refer to the periodic table and the polyatomic ion resource.
The theoretical mass of sodium sulfate is
grams.
dmentum. All rights reserved.
@
2
#
$
%
3
4
5
9
Reset
Next
וום
&
*
7
8
W
e
r
t
Y
u
S
d
f
g
9
吆
Jun
P
P
hiki
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Sakai : CHM 151 170 FA21: Exan x -> b sakai.durhamtech.edu/portal/site/2021fa-chm-151-170/tool/5f0a6298-e555-44a9-9a8f-40c43992fa29/jsf/delivery/deliverAssessment Sakai : My Workspa. www.cfnc.org | 520. + Scholarships - Goo. C Going Merry - Brow.. A Gates: Sign Up wh ccsf Application Status f. A MyMountaineer E Reading list >> The pressure of carbon dioxide gas in a sealed 2.5 L flask is 2.1 atmospheres. Calculate the new pressure of carbon dioxide if all gas was transferred to a 7.5 L flask. OA 6.3 atm O B. You need to know the number of moles and the temperature of CO, to perform this calculation. OC 0.70 atm OD. 2.1 atm OE 4.2 atm Reset Selection Previous Next Save O EXTD V O 5:54 DELLarrow_forwardGiven the following data in the Determination of Molar Mass of CO2 lab : Mass (before rxn): test tube + HCl(aq) + stir bar + capsule Mass (after rxn): test tube + HCl(aq) + stir bar + capsule Volume of water displaced from squirt bottle Temperature of CO₂(g) 26.611 g 25.783 g 144 mL CO2(g) pressure Gas constant R 293.6 K 0.986 atm 0.08206 L'atm/mol.K a) Calculate the mass of CO₂(g) produced, in grams. b) Calculate the density of CO₂(g) in g/L. C) Calculate an experimental value for the molar mass of CO2 based on the mass, volume, and temperature data from the Determination of Molar Mass of CO₂ lab. d) Calculate the number of moles of CO₂(g) produced. Use the accepted value for the molar mass of CO2.arrow_forward69 Gpa, = 0.35)is loaded by an axiel force of 3N, m24mm 12mm 15mm (al Delermine he avetuge ponnal stress ln sechon AB and BC of the chaft at locations away from thefillet Section AB, = Section BC, o rid (O)Determine the change of thee diameter in seien AD due to Polsson's effect. Section AB, Ad%= (c) Determine te hrbest nonnd stress al the filt consideing sTnss concentration. Athe fillet, o= (d) Determine the nommal and shear stresses on the oblique surface that has an inxline angle of Fwiththe nomal cross secbon of the shaft in section AB as shown. pwa the otliquearrow_forward
- -> https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQUHIQg6bJxmeSyVpHOEB1plef9xyC5Ca9QI15ULF571w... A to tho subject) - maryhan X M <3- mary.hamilton@s X To 3 1 O ADVANCED GENERAL CHEMISTRY Calculating an equilibrium constant from an equilibrium.. 3/5 Hydrogen and chlorine react to form hydrogen chloride, like this: H,(g) + Cl,(g) → 2 HCl(g) Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen, chlorine, and hydrogen chloride has the following composition: compound pressure at equilibrium H2 62.7 atm Cl 35.7 atm HCl 85.0 atm Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. d. K D x10 Eſplanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Usel Privacy Center | Acce P W F7 F8 F9 F10 F11 F12 PriScr risert Delete FA FS F6arrow_forwardapp.101edu.co Inbox (17)-ilodya... in Elms College Moo... < Book Pro 1 4 7 +/- A sample of a gas occupies 335 mL at 18.3 °C. How many ml will it occupy at 55.3 °C? 2 LO 5 Aktiv Chemistry a... 8 Question 10 of 14 3 6 9 0 ☐ calculus-10th-edit... x 100 Paused Submitarrow_forwardA www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IQİHQRDYV_6Ux63SypJXz0Coxvwqgg4JkWI7419gav-Tdu6vMUuy4G2alolbmyyMNM8IDN. O STOICHIOMETRY Solving for a reactant in solution Jacqueline v One way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is by titrating a sample of silver nitrate solution. Any chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. Suppose an EPA chemist tests a 250. mL sample of groundwater known to be contaminated with iron(II) chloride, which would react with silver nitrate solution like this: FeCl,(aq) + 2 AgNO3(aq) → 2 AgCl(s) + Fe(NO,),() The chemist adds 32.0 mM silver nitrate solution to the sample until silver chloride stops forming. He then washes, dries, and weighs the precipitate. He finds he has collected 7.4 mg of silver chloride. Calculate the concentration of iron(II) chloride contaminant in the original groundwater sample. Round your…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY