
Ionic compound: Ionic compound consists of cation which is positively charged (+charge) and an anion which is negatively charged (-charge).
Ionic compounds are named by writing the name of the cation first, followed by the anion.
If a metal can form cations with more than one charge, they are named accordingly with the use of roman numerals in parentheses.
Empirical formula: It can be defined as the simplest whole number ratio of different atoms present in the compound.
To find the empirical formula of an ionic compound, first identify the cation and write down its symbol and charge.
Then, identify the anion and write down its symbol and charge.
Finally, combine the two ions so that they form an electrically neutral compound.
Step by stepSolved in 3 steps with 2 images

- please answer quickly thank u (:arrow_forwardPlease don't provide handwritten solution.....arrow_forwardOWLV2 | Online teachin X According To The Following+ My Home gagenow.com/ilm/takeAssignment/takeCovalentActivity.do?locator=assignment-take&takeAsignmentSessionLocator=assignment-take [Review Topics] [References] E 2req Use the References to access important values if needed for this question. 3 2reg For the following reaction, 0.397 grams of hydrogen gas are allowed to react with 54.1 grams of iodine. S 2req hydrogen(g) + iodine(s) – → hydrogen iodide(g) What is the maximum mass of hydrogen iodide that can be formed? grams What is the FORMULA for the limiting reagent? Es 2req is 2req What mass of the excess reagent remains after the reaction is complete? grams ts 2req ts 2req Submit Answer Retry Entire Group 9 more group attempts remaining ts 2req ots 2req ots 2reg ots 2req ots 2req pts 2req Previor Show Hint a $ hp Ins prt sc f9 12 delete 16arrow_forward
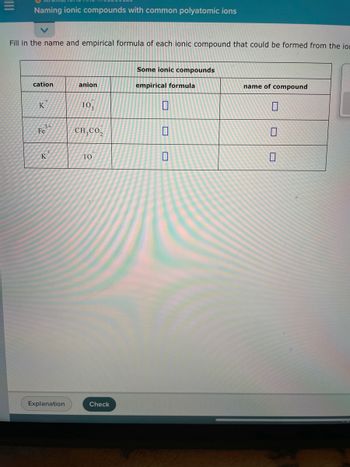
- Complete the table below for calculating the molar mass of the compound phosphorus triiodide. Molar mass of element Mass in one mole of phosphorus triiodide Moles g/mol mol %3D g g/mol x mol %3D Molar mass phosphorus triiodide = g/molarrow_forwardWrite the empirical formula for at least four ionic compounds that could be formed from the following ions: NH4, Fe³+, C₂H₂0₂, MnO4 0 0.0....arrow_forwardFill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation anion 3+ Fe 2+ Fe Cs Sp. 2+ CI CI CI CI Some ionic compounds empirical formula 00 name of compound 0 X 00arrow_forward
- please answer quicklyarrow_forwardWrite the empirical formula of at least four binary ionic compounds that could be formed from the following ions: Fe, AI", F, 0- 0.0.arrow_forwardChromium forms four different oxides in which the percent by mass of chromium is respectively 76.5%, 68.4%, and 52%. Find the formula and give the names of the first - fourth oxides.arrow_forward
- Complete the table below for calculating the molar mass of the ionic compound manganese(II) fluoride . Formula Molar mass of jon Number of ions Mass of ion in one mole of manganese(II) fluoride Cation Mn2+ g/mol x mol g Anion g/mol mol Molar mass manganese(II) fluoride = g/molarrow_forwardGive detailed Solution (no need Handwritten answerarrow_forwardWrite the empirical formula for at least four ionic compounds that could be formed from the following ions: PO, NH, I03, Fe2- Fe²* 4 ! (NH,), (PO,), O (РО, 4 0,0,...arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





