Carbon tetrachloride (CCl4) is diffusing through benzene (C6H6), as the drawing illustrates. The concentration of CCl4 at the left end of the tube is maintained at 1.09 x 10-2 kg/m³, and the diffusion constant is 23.4 x 10-10 m²/s. The CCl4 enters the tube at a mass rate of 4.38 x 10-13 kg/s. Using these data and those shown in the drawing, find (a) the mass of CCl4 per second that passes point A and (b) the concentration of CCl4 at point A. 5.00 x 10-³ m -CCI4 Cross-sectional area = 3.00 x 10-4 m²
Carbon tetrachloride (CCl4) is diffusing through benzene (C6H6), as the drawing illustrates. The concentration of CCl4 at the left end of the tube is maintained at 1.09 x 10-2 kg/m³, and the diffusion constant is 23.4 x 10-10 m²/s. The CCl4 enters the tube at a mass rate of 4.38 x 10-13 kg/s. Using these data and those shown in the drawing, find (a) the mass of CCl4 per second that passes point A and (b) the concentration of CCl4 at point A. 5.00 x 10-³ m -CCI4 Cross-sectional area = 3.00 x 10-4 m²
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter16: Temperature And The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 39P
Related questions
Question
100%
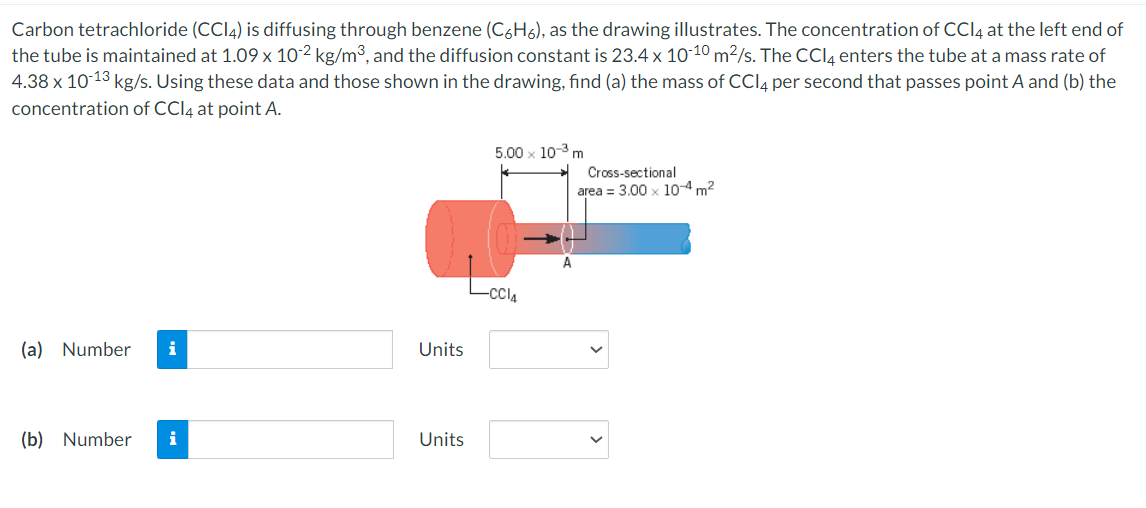
Transcribed Image Text:Carbon tetrachloride (CC14) is diffusing through benzene (C6H6), as the drawing illustrates. The concentration of CCl4 at the left end of
the tube is maintained at 1.09 x 10-2 kg/m³, and the diffusion constant is 23.4 x 10-10 m²/s. The CCl4 enters the tube at a mass rate of
4.38 x 10-13 kg/s. Using these data and those shown in the drawing, find (a) the mass of CCl4 per second that passes point A and (b) the
concentration of CCl4 at point A.
(a) Number i
(b) Number i
Units
Units
5.00 x 10-³ m
-CCI4
Cross-sectional
area = 3.00 x 10-4 m²
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning
