
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Ff.191.
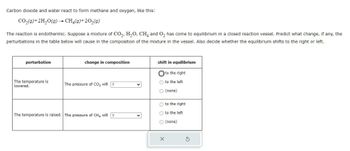
Transcribed Image Text:Carbon dioxide and water react to form methane and oxygen, like this:
CO₂(g) + 2H₂O(g) → CH₂(g) +20₂(g)
The reaction is endothermic. Suppose a mixture of CO₂, H₂O, CH4 and O₂ has come to equilibrium in a closed reaction vessel. Predict what change, if any, the
perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left.
perturbation
The temperature is
lowered.
change in composition
The pressure of CO₂ will ?
The temperature is raised. The pressure of CH4 will ?
v
shift in equilibrium
to the right
O to the left
O (none)
O to the right
O to the left
O (none)
X
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Every year Every second (1 year 365 days).arrow_forwardAn aqueous solution of sodium chloride,NaCl , is made by dissolving 1.01 grams of sodium chloride in sufficient water in a 50.0 mL volumetric flask, and then adding enough water to fill the flask to the mark. What is the weight/volume percentage of sodium chloride in the solution? Weight/volume percentage = %arrow_forwardA student sets up the following equation to convert a measurement.(The ?stands for a number the student is going to calculate.)Fill in the missing part of this equation. Note: your answer should be in the form of one or more fractions multiplied together.arrow_forward
- H8.arrow_forwardFor the reaction 2A(g) s B(g) K. = 69 For the reaction A(g) = ½ B(g) Kc = For the reaction ½ B(g) A(g) Kc = Report each answer to two significant figures. Do NOT use scientific notation.arrow_forwardMany common materials that we ingest, though quite safe in reasonable quantities, become toxic when taken in very large doses. A measure of toxicity is the LD50 value (Lethal Dose, 50%). It is the quantity of material, expressed in mg of material per kg of subject-body-weight that, if administered to a population of subjects, would cause 50% of the population to die. The LD50 value for FD&C Red Dye No. 40 is >10,000 mg/kg in rats. Assume that the LD50 value for humans is the same as for rats. Calculate the number of mg of Allura Red present in an 8 fluid ounce glass of the beverage you used in this lab. Assume that the concentration of Allura Red in the beverage is 0.000054 M. The molar mass of Allura Red is 496.42 grams/mol 1 fl oz = 29.5735 mL Do not include units with your answer.arrow_forward
- A student synthesizes tin oxide to create a compound with generic formula SnxOy. She does this by combining 30.80 g of tin and when the tin oxide product is produced, the total mass of the product is 39.10 g. Consider the starting mass of the tin (Sn) and the ending mass of the SnxOy product. To find the mass of oxygen in the product, you would _____ A) add the mass of tin from the mass of the product. B) subtract the mass of tin from the mass of the product. C) multiply the mass of tin from the mass of the product. D) divide the mass of tin from the mass of the product.arrow_forwardThe world population is estimated to be 7.4 x 109. Nauru is the smallest island nation and comprises 1.5 x 10-4% of the world population. If the percentage of left-handed people is approximately 12%, estimate the number of left-handers on the island of Nauru.arrow_forwardThe number of oxygen atoms in 20.18 grams of Al(NO;); is: O 1.712 x 1023 O 0.09474 5.135 x 1023 O 5.705 x 1022arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY