
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Can someone help with this Mechanism question? Please make sure to include all curved arrow notation and to include all resonance structures so I can learn this please.
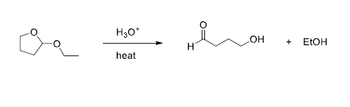
Transcribed Image Text:H3O+
heat
OH
+ EtOH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please correct answer and don't use hend raitingarrow_forwardFor molecule showed on the rightdraw the structure of 3 different conjugated basesbased on 3 different types of hydrogen which could be deprotonated (CH3, and two different CH2groups). For each structure of conjugate base draw all possible resonance structuresand finally rank all 3 C-H bonds in order of increasing their acidity.arrow_forwardWhy was the formal charge of (+) placed on this carbon? I understand everything else about the question but can someone help me with explaining this. Thanks!arrow_forward
- Solve correctly please, with detailed explaination. When solving alkene addition reactions, how do you know which double bond undergoes the addition reaction if there is more than one? (Gpt/Ai wrong answer not allowed)arrow_forward4. Which of the following species are aromatic or anti-aromatic (assuming n-systems are fully planar)? Circle those that are aromatic Put an X through those that are anti-aromatic Do nothing to the structure otherwise 5. Which of the following compounds will have the larger Amax ? Briefly explain in terms of MO theory and photon energy & basic wave property equations. 6. For the allyl anion below, A) Draw the second resonance structure and show the arrow-pushing needed to get to that structure. B) Draw the Valence Bond Theory Orbital Overlap Diagram of the resonance hybrid C) Make an Aufbau diagram of the relative energies of the n molecular orbitals, distribute the electrons of the p orbitals to the MO's, and label the MO's as r, mb, and ri (molecular orbital theory) D) Sketch the shapes of the 3 molecular orbitals in part C.arrow_forwardGive typed explanationarrow_forward
- Click the "draw structure" button to launch the drawing utility. Draw a second resonance structure for the following radical. EGO draw structure ...arrow_forward13. Aniline and nitrobenzene are two substituted benzene compounds that contain nitrogen atoms. A) Use resonance structures to identify all carbon atoms that are electron rich on aniline. Mark the appropriate carbons in the figure below with a d-. NH₂ nitrobenzene aniline Note: this is benzene B) Use resonance structures to identify all carbon atoms that are electron deficient on nitrobenzene. Mark the appropriate carbons in the figure below with a dª.arrow_forwardFirst, add curved arrow(s) to show the resonance using the following pattern: an allylic lone pair. Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to increase or decrease the charge on an atom, and use the single bond tool to add/remove double bonds. What am I missing here? I've tried putting charges in various spots and still can't get it.arrow_forward
- Please solve b part and explain it too....if you won't I will downvotearrow_forwardPlease help answer the attached question...thank you!arrow_forwardplease do all 3 , very easy question, do in a neat and clean white paper , do not give incomplete solution, if u plan to do only 1 then skip , someone will do all, i will upvote plz do allarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY