
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
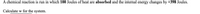
Transcribed Image Text:A chemical reaction is run in which 100 Joules of heat are absorbed and the internal energy changes by +398 Joules.
Calculate w for the system.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate AS for NH3(g). AS = i Use data in the table below: Substance Ag(s) AgCl(s) Al(s) Al₂O3(s) C(s) (graphite) CO(g) CO₂(g) CH₂(g) CH₂Cl(g) CH₂OH(1) C₂H₂(g) C₂H₂(g) C₂H6(g) CgH 18(1) C₂H₂OH(1) Standard Entropies of Some Typical Substances at 298.15 K Sᵒ (J mol-¹ K-¹) 40 76.1 Ca(s) CaCO3(s) SaCl₂(s) Sº (J mol-¹ K-¹) 42.55 96.2 28.3 51.0 186.2 234.2 126.8 CO(NH2)2(s) 104.6 CO(NH,)2(aq) 173.8 200.8 219.8 229.5 466.9 161 5.69 197.9 213.6 41.4 92.9 114 JK-1 Substance CaO(s) Ca(OH)₂ (s) CaSO4(s) 107 CaSO4+H₂O(s) 131 CaSO4-2H₂O(s) 194.0 223.0 27 90.0 130.6 188.7 Cl₂(g) Fe(s) Fe₂O3(s) H₂(g) H₂O(g) H₂O(l) HCI(g) HNO3(1) H₂SO4(1) HC,H,O,(D) Hg(1) Hg(g) K(s) KCl(s) K₂SO4(s) 69.96 186.7 155.6 157 160 76.1 175 64.18 82.59 176 Substance N₂(g) NH3(g) NH₂Cl(s) NO(g) NO₂(g) N₂O(g) N₂O4(g) Na(s) Na₂CO3(s) NaHCO3(s) NaCl(s) NaOH(s) Na₂SO4(s) 0₂(8) PbO(s) S(s) SO₂(g) SO3(g) Sº (J mol-¹ K-¹) 191.5 192.5 94.6 210.6 240.5 220.0 304 51.0 136 102 72.38 64.18 149.4 205.0 67.8 31.9 248.5 256.2arrow_forward2.18 A mole of argon is allowed to expand adiabatically and reversibly from a pressure of 10 bar and 298.15 K to 1 bar. What is the final temperature, and how much work is done on the argon?arrow_forwardSuppose we're interested in studying ligaments. In class, we have focused on Problem 1 work done by changes in volume, but our initial framework presented accommodates other forms of work as well. When a ligament is stretched, we may represent the work done on the system as follows: dw fdl - pdV || Here, fis the restoring force of the ligament that opposes stretching, and /is the length. The differential expression for the Gibb's free energy can be written as follows: dG = Vdp – SdT + fdl differential expression. dA. a) Write all Maxwell relations from the Gibb's free energy energy, b) Derive the differential expression for Helmholtz freearrow_forward
- Beginning from the Clausius inequality, derive for us several (two or more) units of entropyarrow_forwardConsider the free, isothermal (constant T) expansion of an ideal gas. "Free" means that the external force is zero, perhaps because a stopcock has been opened and the gas is allowed to expand into a vacuum. Calculate AU for this irreversible process. Show that q sion is also adiabatic (q = 0) for an ideal gas. This is analogous to a classic experiment first performed by Joule. 0, so that the expan-arrow_forward6. In compressing a gas at constant pressure, work is done on the system and the internal energo increases. The work done is given by P times AV, where AVValmunal Note that since the is smaller than Vital In calculating the change in internal energy, which gas is compressed, Vinal is smaller than V of the following expressions should be used? Explain. a) w = PAV b) w=-PAVarrow_forward
- A 1 mol sample of an ideal gas with a constant volume heat capacity of 41.6 J/K is at 4.25 atm and 300K. After completing a reversible, adiabatic expansion the pressure reaches 1 atm. Calculate the following i) final volume ii) final temperature iii) the work donearrow_forwardTrue or False. Support your answer.arrow_forwardYou have four distinguishable particles held inside a container. Calculate the number ofmicrostates for each of the following states of the system:A. all of the particles are in the left half of the containerB. two of the particles are in the left half and two are in the right halfC. three particles are in the left half and one particle is in the right halfD. What does this suggest about the relative entropies of each state (A, B and C)?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY