
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Calculate theoretical yield of the Grignard reaction synthesis of triphenylmethanol from bromobenzene. Amounts of products used are listed in the image.
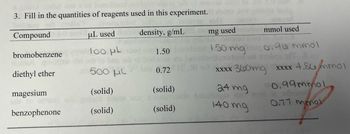
Transcribed Image Text:3. Fill in the quantities of reagents used in this experiment.
Compound
density, g/mL
bromobenzene
A que
diethyl ether
magesium
to stud
benzophenone
uL used
100 μL
500 ML
(solid)
gntivo sbsm NO
(solid)
1.50
0.72
(solid)
noiievised
(solid)
mmol used
mg used
150 mg 0.96 mmol
nodne not smAq@T, &.01
xxxx 360mg xxxx 4.86 mmol
0.99mmol
16 odimos
24 mg
140 mg
0.71 mmol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following set of chloride on seperate all quots Of a pette pooled Serum obtaned from SMu chemistry lab here reporterd by (43) students : (03:106; (07 and 114 meqlL. analysis One value qppears suspect. Help these Students to determine if it can be ascribed to the acudental 95% Confidence level. error otarrow_forwarding 0.03% w/w_of the same drug, who would be the percentage strength (wh) a of of the mixture? 23. A physician prescribes an suspension to contain 100 mg of corti- at n ophthalmic st sone acetate in 8 mL of normal saline solution. The pharmacist has on hand a (2.5% suspension of cortisone acetate in normal saline solution. How many milliliters of this and how many milli- le S liters of normal saline solution should b be used in preparing the prescribed sus- pension?gorq ww.Ps-3/9rie 24. R Benzalkonium Chlordaarrow_forwardChromatography question A student properly sets up a column chromatography experiment. Although the mixture is soluble i the mobile phase, the sample appears "stuck" at the top of the column and will not elute eregardless of how much solvet is pplaied. what could bee the cause of this issue and what could teh student try to achieve a successful separation?arrow_forward
- QUESTION T5 Why were the areas underneath the peaks in the gan chromatographs you eobtained mutiplied by a weight tactor? DA the corrction factor compensates for user error OB Rame lonization detectors (FID) respond slightly dfferent to compounds with diferent molecular weights OCnone of the answers OD. the GC (GOW MAC 350) used contains a quadrapole mass spec detector whose response must be corrected for the miz ratio of the diferent ions obtainen for diferert moleculestune OE not all molecules have the same themmal conductivity QUESTION 16 Which list of functional groups is present in the anti-viral drug Arbidol HO Br O A Thioether, alkene, ester, amide, amine O 8. Thioether alcohol, ester, amine OC Alcohol, ether, amide, epoxide, thioester OD. Ether, ketone olefin, amine, alcohol O E Thioalcohol, alkene, ether, amine, nitroarrow_forwardPlease answer very soon will give rating surelyarrow_forward119 X My Course X Macmillan: X Course Mo X What is the FORMULA for the limiting reagent? Submit Answer Sections 5 X .com/static/nb/ui/evo/index.html?deploymentid=5735112480241329813180832311&elSBN=9781305862883&id=1707786042&snapshoti... Use the References to access important valdes ir needed for this question. 900 For the following reaction, 9.43 grams of carbon (graphite) are allowed to react with 28.5 grams of oxygen gas. carbon (graphite) (s) + oxygen (g) carbon dioxide (g) What is the maximum amount of carbon dioxide that can be formed? What amount of the excess reagent remains after the reaction is complete? HOMEWOR X MacBook Air MindTap - C X GI grams gramsarrow_forward
- What is the key equation for spectrometry experiments? O q rxn = -q_cal %3D O d=m/V O A= ebC O at endpoint, mol acid = mol basearrow_forwardhow do i calculate the Theoretical and % yield using this table?arrow_forwardoverall aden fn tereactiorabwe! .20 what is te overall aae for tereactrobre! 200 0.00 8.00xl0 1. 0. 1000 200x1o? Trual A +BSAB On 12arrow_forward
- How can we answerarrow_forwardWith correct sig figsarrow_forwardIn a chromatography experiment, a solution containing 0.083 7 M X and 0.066 6 M S gave peak areas of AX = 423 and AS 5 347. (Areas are measured in arbitrary units by the instrument.) To analyze the unknown, 10.0 mL of 0.146 M S were added to 10.0 mL of unknown, and the mixture was diluted to 25.0 mL in a volumetric flask. This mixture gave the chromatogram in Figure 5-8, with peak areas AX = 553 and AS = 582. Find the concentration of X in the unknown.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

