
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
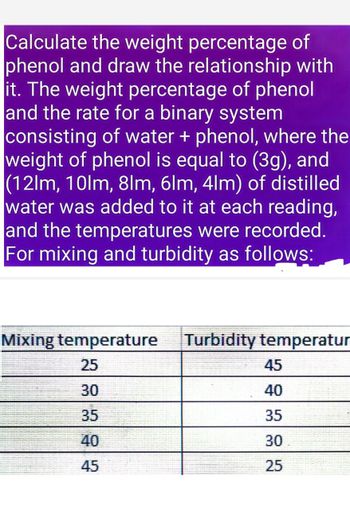
Transcribed Image Text:Calculate the weight percentage of
phenol and draw the relationship with
it. The weight percentage of phenol
and the rate for a binary system
consisting of water + phenol, where the
weight of phenol is equal to (3g), and
(12lm, 10lm, 8lm, 6lm, 4lm) of distilled
water was added to it at each reading,
and the temperatures were recorded.
For mixing and turbidity as follows:
Mixing temperature Turbidity temperatur
25
45
30
40
35
35
40
30
45
25
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 14 images

Knowledge Booster
Similar questions
- 4. Pure octane has a boiling point of 125.7 °C, but can be steam distilled with water at a tem- perature of 90 °C. Calculate the mass of octane that codistills with each gram of water and the percent composition of the vapor that is produced during the steam distillation. [Again, you'll need the vapor pressure of water at the steam distillation temp to solve this problem!] Note that if you multiply the second equation from question 3 above by the MW of both the oil and water, you get: mass oil/mass water = (P°) (MW)/ (Pwater) (MW water)arrow_forwardtype solution in wordarrow_forwardCalculate the bubblepoint temperature (in °F) of a liquid mixture containing 60 mol% n-butane, 25mol% n-pentane, and 15% n-hexane at 150 psia.arrow_forward
- number 10arrow_forwardWe are tasked to do iteration in mass and energy balances, wherein we will look for the final temperature. When there are two components, like CO and CO2, do you add their enthalpy out (i.e. 3.376 + 0.557x10-3T - 0.031x105T-2 for CO and 5.457 + 1.045x10-3T - 1.157x105T-2 for CO2) to use for the calculation of Cp, mean? If yes, what would be the value of Cp, mean?arrow_forwardUsing the provided ternary phase diagram 1) Determine the EXACT composition of point a2. 2) Determine the maximum amount of (in moles) of pure E that can be added to a 10 mol mixture at point a2 to maintain a two-phase system?arrow_forward
- Calculate the vapor phase mole fractions in equilibrium with a liquid hydrocarbon binary mix of 42% carbon tetrachloride and 58% trichloroethylene (use Antoine equation to calculate vapor pressures), for a system at 60° Also, calculate what is the total system pressure?arrow_forward1 Temperature (°C) 110 100 90 80 mol % Toluene 0 mol % Benzene 100 20 80 Vapor line 40 60 60 40 Composition (mol %) Liquid line 80 20 100 0 If the composition in (d) is condensed what would be more enriched compared to the mixture of the 50 mol % benzene and 50 mol % toluene?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The