
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
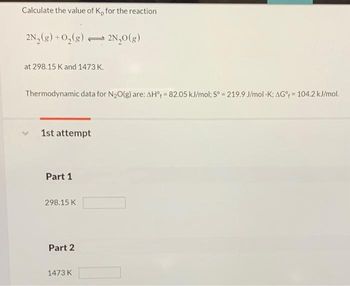
Transcribed Image Text:Calculate the value of K, for the reaction
2N₂(g) + O₂(g) 2N₂O(g)
at 298.15 K and 1473 K.
Thermodynamic data for N₂O(g) are: AH = 82.05 kJ/mol; Sº = 219.9 J/mol -K; AG% = 104.2 kJ/mol.
1st attempt
Part 1
2
298.15 K
Part 2
1473 K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the value of Kp for the reaction at 298.15 K and at 1173.00 Karrow_forwardConsider the following thermodynamic table. S° (J/mol-K) H (kJ/mol) G (kJ/mol) . Ca(OH)2 (s) 83.4 -986.1 -898.6 Ca2+ (aq) -53.1 -542.8 -553.6 OH (aq) -10.8 -230.0 -157.2 Which of the following statements is correct with respect to the reaction Ca(OH), (s) = Ca2+ (aq) + 2 OH (aq) ? O a) The reaction is endothermic. The solubility of calcium hydroxide will increase with increasing temperature. b) The reaction is exothermic. The solubility of calcium hydroxide will decrease with increasing temperature. c) The reaction is endothermic. The solubility of calcium hydroxide will decrease with increasing temperature. O d) The reaction is exothermic. The solubility of calcium hydroxide will increase with increasing temperature.arrow_forward76. Consider the reaction: CO₂(g) + CCL (g) = 2 COC1₂ (g) Calculate AG for this reaction at 25 °C under the following conditions: Pco₂ = 0.112 atm; Pccl 0.174 atm; Pcocl₂ 0.744 atm.. =arrow_forward
- From data given below, calculate AH°, AS°, and AG° for the following reaction at 25°C. CH4 (g) + 202(g) → CO2(g) + 2H2O(g) AH -75 kJ/mol AG -51 kJ/mol 186 J/K mol -393.5 -242 -394 -229 S° 205 214 189 ΔΗ' kJ AS J/K AG° = kJarrow_forwardUsing Le Chatelier's Principle to predict the result of changing... Ammonia and oxygen react to form nitrogen and water, like this: 4NH3 (g)+30₂ (g) 2N₂(g)+6H₂O(g) Suppose a mixture of NH3, O2, N₂ and H₂O has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation Some NH3 is added. Some H₂O is removed. change in composition The pressure of O₂ will The pressure of N₂ will The pressure of NH3 will ? ? ? shift in equilibrium to the right to the left O (none) to the right to the leftarrow_forwardThe reaction A + B <> C has a molar DSrxn of -19 J/K and a molar DHrxn of 15 kJ. C has a free energy of formation of 10,000 kJ/mole, and B's free energy of formation is -3500 kJ/mole. What is the free energy of formation of A? Assume standard conditions, choose the closest. -7200 kJ/mole -5700 kJ/mole 7200 kJ/mole 5700 kJ/mole -34100 kJ/molearrow_forward
- 12. Calculate the Gibbs free energy (in kJ) at 39 degrees C if ΔH reaction is -165 kJ/mol and ΔS reaction is 292 J/K.arrow_forwardConsider a chemical reaction where AS is 40.0 J/K, and AH is 25.0 kJ. What is the change in free energy (AG) for this reaction at 298K? O-22.4 kJ 3.9 kJ O 65.9 kJ O-37.9 kJ O 13.1 kJarrow_forwardThe thermite reaction produces tremendous heat:2 Al + Fe2O3 → Al2O3 + 2 Fe ΔrH° = – 851.5 kJ/molHow much heat is released when 24.0 g of Al reacts with excess Fe2O3 at constant P ?arrow_forward
- Calculate the equilibrium temperature (in K, to the nearest whole number) for the illustrated closed system, assuming the mass of the copper block is equal to the mass of the silver block. Silver Сopper c,=0.240 J/g °C c,=0.385 J/g °C 264 K 436 Karrow_forwardConsider a reaction in the gas phase. The equilibrium constant, K, is found to be independent of the temperature. What does this tell you about ∆rH0 of the reaction?arrow_forwardCalculate ΔH°, ΔS° and ΔG° for the synthesis of SO3(g) Be sure to use the values provided in this table: Compound ΔHf° (kJ/mol) S° (J/Kmol) SO2(g) -253 231 SO3(g) -345 219 O2(g) 0 205 a) give the balance equation for the synthesis of SO3(g) from SO2(g) and O2(g) b) What is ΔH° for this process? c) What is ΔS° for this process? d) What is ΔG° for this process?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY