
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
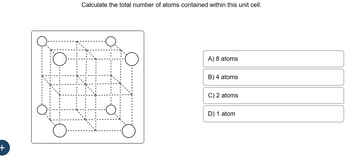
Transcribed Image Text:**Title: Calculating Atoms in a Unit Cell**
**Question:**
Calculate the total number of atoms contained within this unit cell.
**Diagram:**
The diagram shows a cubic unit cell structure with atoms represented by circles. The vertices of the cube and the centers of the cube faces are occupied by atoms. This configuration typically represents a face-centered cubic (FCC) lattice.
**Answer Choices:**
A) 8 atoms
B) 4 atoms
C) 2 atoms
D) 1 atom
**Explanation:**
In a face-centered cubic (FCC) lattice:
- Each corner atom is shared by 8 adjacent unit cells.
- Each face-centered atom is shared by 2 adjacent unit cells.
- There are 8 corner atoms (each contributing 1/8th of an atom) and 6 face-centered atoms (each contributing 1/2 of an atom).
To calculate the total number of atoms in one FCC unit cell:
- Contributions from corner atoms: \(8 \times \frac{1}{8} = 1\) atom
- Contributions from face-centered atoms: \(6 \times \frac{1}{2} = 3\) atoms
- Total number of atoms: \(1 + 3 = 4\) atoms
Thus, the correct answer is **B) 4 atoms**.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Refer to the following physical model to answer the questions. White Sulfide ion Blue Zinc ion Download or redraw the image below, indicating the positions of the atoms in a ZnS Unit Cell OOOO 1&5 2 3arrow_forwardCrystal structure Worksheet 1. NaCl has a has a face centered cubic (fcc) structure. a. How many (a) Na atoms per unit cell, Cl atoms per unit cell and (c) total atoms (Naplus CI) per unit cell for NaCl?arrow_forwardA substance is made of elements X, Y and Z. In the unit cell of the compound, X atoms occupy corners of the unit cell, Y atoms occupy center of faces of unit cell and Z atoms occupy edges of the unit cell. Determine the simplest formula of the substance. A. XY3Z4 B. XY3Z C. XY2Z3 D. XY3Z2 E. XY3Z3arrow_forward
- The density of Rh is 12.4 g/mL and the cell volume is 5.50 x 10-23 cm3. Determine the number of atoms in the unit cell. Note: the number of atoms in a unit cell is a whole number.arrow_forwardSolid strontium chloride has the same kind of crystal structure as CaF2 which is pictured below:fc396f75-defe-4edf-a700-e8a3e755e3aa.GIFHow many Cl- ions are there per unit cell in solid strontium chloride? How many SrCl2 formula units are there per unit cell?arrow_forwardPart A The unit cells for lithium oxide and silver iodide are shown below (Figure 1). Show that the ratio of cations to anions in the unit cell of Li,0 corresponds to the ratio of cations to anions in the formula of the compound. Match the numbers in the left column to the appropriate blanks in the sentences on the right. Reset Help 1 The Li atoms occupy position(s) inside the unit cell, giving 2 Li atom(s)/unit cell. 3 Figure < 1 of 1 The O atoms occupy corner 4 position(s) and face-center 5. position(s) of the unit cell, giving O atom(s)/unit cell. The ratio of Li :0 atoms is 8 which reduces to 10arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY