
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
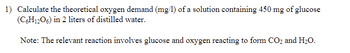
Transcribed Image Text:1) Calculate the theoretical oxygen demand (mg/l) of a solution containing 450 mg of glucose (C₆H₁₂O₆) in 2 liters of distilled water.
Note: The relevant reaction involves glucose and oxygen reacting to form CO₂ and H₂O.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Describe the process by which the ionic compound, LiCl, would dissolve in the polar solvent, CH3COCH3.arrow_forwardA 1 mL sample of glycogen was calculated to contain 21 µmol (micromole) glucose. To 1 mL of this sample was added 2 mL of 2 M HCl. It was then hydrolysed by boiling the solution for 15 minutes. After boiling the hydrolysate was cooled and made up with H2O to a final volume of exactly 10 mL. The glucose was measured in this solution and found to have a concentration of 340 µg/mL (microgram/milliliter). i) Calculate the mass (mg) of glucose in the 10 mL of hydrolysate. As the 1 mL of glycogen sample was made up to a final volume of 10 mL, this mass of glucose was produced by the hydrolysis of the original 1 mL glycogen sample. ii) Calculate the amount (µmol) of glucose produced by the hydrolysis of the glycogen sample. iii) Calculate the purity of the glycogen used in the sample as % Purity = (moles of measured glucose/ moles of calculated glucose in glycogen) *100 iv) state your answer in a complete sentence. Show your working out such that the marker can easily understand it.…arrow_forwardThe label on a jar of jam says it contains 13 g of sucrose, C12H22O11 per tablespoon (15 mL). What is the molarity of sucrose in the jam?arrow_forward
- The label on the jar of a jam says it contains 13.0 g of sucrose (C12H22O11) per tablespoon (15 mL). What is the molarity of sucrose in the jam?arrow_forward0.500 g of an unknown nonelectrolyte are dissolved in 25.0 g water. The resulting solution froze at-0.619 °C. Calculate the molar mass of the unknown. Kf (H2O) = 1.86 °C/m O 0.00416 g/mole O 120 g/mole O 0.00832 g/mole O 60.1 g/mole O 30.1 g/molearrow_forward(please type answer).arrow_forward
- What is the molarity of an aqueous solution of glucose (C6H12O6) 0,433 mol/kg? The density of the solution is 1,16g/mLarrow_forwardWhat is the molarity of a solution made by dissolving 1.250 grams of NazCo3 in H20 to make 100.0 mL of solution?arrow_forwardWhat molaity of pentane is obtained by dissolving 5g pentane, C5H12, in 245 g hexane C6H12arrow_forward
- CALCULATE the molarity of NaI in a solution obtained by adding 5 mL of 0.2M NaI to a mixture of 5 mL of 0.2M of K2SO4, 5 mL of K2S2O8, 4mL of H2O and 1 mL of starch.arrow_forwardWhat mass of sucrose, C12H22O11, must be dissolved per liter of water (d= 0.998 g/ml) to obtain asolution with 2.75 mole percent C12H22O11?arrow_forwardWhat is the molarity of a 10.5% by mass glucose (C6H12O6) solution? (The density of the solution is 1.03 g/mL.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY