
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Calculate the standard free energy for the reaction (you may use any valid technique - there may be more than one way to do a question like this):
Al2O3(s)) + 3 H2(g) → 2 Al(s) + 3 H2O(g)
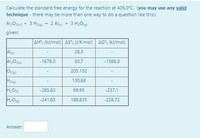
Transcribed Image Text:Calculate the standard free energy for the reaction at 405.0°C. (you may use any valid
technique - there may be more than one way to do a question like this):
Al>O36) + 3 Hzig) - 2 Als) + 3 H20g)
given:
AH; (kJ/mol) AS; (J/K-mol) AG"; (kJ/mol)
28.3
-1588.0
Al2O3)
50.7
-1678.0
Ozig)
205.152
Hzig)
130.68
-285.83
69.95
-237.1
-241.83
188.835
-228.72
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chemistry Small amounts of Fe(CO)5(l) usually form in steel tanks containing pressurized CO(g). You worked out the thermodynamics of this in question 1b. At what temperature (oC) will the formation of Fe(CO)4 become non-spontaneous? Show your work. b) A similar reaction occurs to make Ni(CO)4(l) with Gorxn = −38 kJ/mol, Horxn = −230 kJ/mol, and Sorxn = −480 J/Kmol. High pressure reactors use a thin disk of metal as a safety mechanism that will rupture and release gasses if the pressure in the reactor gets too high. If one was using CO gas, which disk (Fe or Ni) would be more likely to prematurely fail due to the metal being dissolved away by CO? Briefly explain why.arrow_forwardPlease give me experts solution with step by step and explanationarrow_forwardConsider the reaction shown to answer the following question. 2CO (g) + 2H2 (g) → CO2 (g) + CH4 (g) AH° = - and AS° = - Which statement below is true? O This reaction is spontaneous at relatively higher temperatures and non-spontaneous at lower temperatures. O There is not enough information to make an assessment. O This reaction is spontaneous at relatively lower temperatures and non-spontaneous at higher temperatures. O This reaction is spontaneous at all temperatures. O This reaction is non-spontaneous at all temperatures.arrow_forward
- The standard reaction free energy AGO = − 1378. kJ for this reaction: 2 CH3OH(g) + 3O₂(g) →2 CO₂(g) + 4H₂O(g) Use this information to complete the table below. Round each of your answers to the nearest kJ. 2 4 ²-CH₂OH(g) + O₂(g) → ²-CO₂(g) + H₂O(g) 3 2 3 reaction 3 -- CO₂(g) + H₂O(g) + H₂O(g) → 2 2CO₂(g) + 4H₂O(g) 1 2 3 CH₂OH(g) + ²/0₂(g) 4 2 2CH₂OH(g) + 30₂ (8) 2 0 46° kJ KJ kJ x10 Śarrow_forwardThe reaction, Fe203(s) + 3C(s) → 2Fe(s) + 3CO(g) AH° = +490.7 kJ, AS° = +541 J-K-1 %3D This reaction is spontaneous only below a certain temperature always spontaneous never spontaneous spontaneous only above a certain temperaturearrow_forwardΣ *00 For a particular reaction, AH = 81.95 kJ and AS = 27.0 J/K. Calculate AG for this reaction at 298 K. AG = %3D What can be said about the spontaneity of the reaction at 298 K? O The system is spontaneous in the reverse direction. O The system is at equilibrium. O The system is spontaneous as written. 8:12 PH (口 四< 11/8/20 F5 F8 F12 PrtScr Insert Delete Backspace $4 % 8. 5. 7. 24 Alt Ctriarrow_forward
- 33. Use the standard free energy of formation data in Appendix G to determine the free energy change for each of the following reactions, which are run under standard state conditions and 25 °C. Identify each as either spontaneous or nonspontaneous at these conditions. MnO2 (s) ⟶ Mn(s) + O2 (g) H2 (g) + Br2 (l) ⟶ 2HBr(g) Cu(s) + S(g) ⟶ CuS(s) 2LiOH(s) + CO2 (g) ⟶ Li2CO3 (s) + H2O(g) CH4 (g) + O2 (g) ⟶ C(s, graphite) + 2H2O(g) CS2 (g) + 3Cl2 (g) ⟶ CCl4(g) + S2Cl2 (g)arrow_forwardA 0.582-g sample of Mg reacts with excess 1.0 M HCl (60.0 mL) according to the procedure used in this experiment. The initial and final temperatures were 24°C and 68°C. What is the ∆H of the reaction per mole of magnesium? Assume that the specific heat capacity for the solution is 4.187 J/oC∙g and that the density of the 1.0 M HCl is 1.00 g/mL. (Hints: the magnesium contributes to the mass of the solution; take care with your sig figs.) (answer: - 4.7 × 102 kJ/mol)Show your solution to this problem in the space below. It is your solution which will be graded.arrow_forwardAcetone, CH3 OCOCH3, is a fragrant liquid that is used as a solvent for lacquers, paint removers, and nail polish remover. It burns in oxygen to give carbon dioxide and water CH: COCH, (1) + 402 (9) → 3CO2(9) + 3H2O(1) If the standard free-energy change for this reaction is –1739.0 kJ/mol, what is the standard free energy of formation of acetone? Substance AG; (kJ/mol) O2 (9) Co (9) НаО() -394.4 -237.1 Standard free energy= kJ/molarrow_forward
- AGO SnO₂(s) + 2 CO(g) → Sn(s) + 2 CO₂(g) Use this information to complete the table below. Round each of your answers to the nearest kJ. The standard reaction free energy reaction 2Sn (s) + 4CO₂(g) == - 194. kJ for this reaction: 2SnO₂ (s) + 4CO(g) 3 SnO₂ (s) + 6CO(g) → 3Sn(s) + 6CO₂(g) Sn(s) + 2CO₂(g) SnO₂ (s) + 2CO (g) AGⓇ ☐ kJ kJ KJ x10 X Śarrow_forwardA chemist fills a reaction vessel with 1.75 atm nitrogen monoxide (NO) gas, 1.94 atm chlorine (Cl₂) gas, and 4.19 atm nitrosyl chloride (NOCI) gas at a temperature of 25.0°C. Under these conditions, calculate the reaction free energy AG for the following chemical reaction: 2NO(g)+Cl₂(g) 2NOCI(g) Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.arrow_forwarddetermine whether each reaction is spontaneous under standard conditions. If a reaction is not spontaneous, write the corresponding spontaneous reaction. K2O2 (s) → 2K (s) + O2(g) PbCO3 (s) → PbO (s) + CO2 (g) P4 (s) + 6H2(g) → 4PH3 (g) 2AgCl (s) + H2S (g) → Ag2S (s) + 2HCl (g)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY