
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
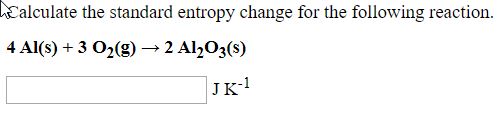
Transcribed Image Text:Calculate the standard entropy change for the following reaction.
4 Al(s) + 3 O2(g) → 2 Al½O3(s)
JK-!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the standard entropy change for the reaction at 25 °C. Standard molar entropy values can be found in this table. HCl(g)+NaOH(s)⟶NaCl(s)+H2O(l) Δ?∘rxn=arrow_forwarda) Briefly describe the Third Law of Thermodynamics. b) Consider the reaction 2X(g)+ O2(g)→3Y(g) carried out at 25°C and 1 atm. Calculate ΔHrxn°, ΔSrxn°, and ΔGrxn° using the following data. Identify whether the reaction is spontaneous or not. (Assume all gases are perfect). Substance DHfo (kJ/mol) So(J/k mol) X (g) -250 250 Y (g) -300 280 O2 (g) 0 205 c) In a reaction, A + B → Product, rate is doubled when the concentration of B is doubled, and rate increases by a factor of 8 when the concentrations of both the reactants (A and B) are doubled. Choose the correct rate law for the reaction from below. Explain your answer briefly. Rate = k[A][B] Rate = k [A][B]2 Rate = k [A]2[B] Rate = k [A][B]3 d) One mole of N2(g) occupies 5 L of volume at 300 K in a sealed cylinder. The gas behaves ideally in expanding isothermally to 10 L. Calculate w, ΔE and ΔH if the expansion proceeds (i) reversibly, (ii) irreversibly against an external pressure at…arrow_forwardGiven the following reaction: 9C (s) + 12H2 (g) + 3/2 O2 (g) → 3C3H7 OH (1) If the enthalpy change is -954 kJ and entropy increases: blank 1: is the reaction exothermic or endothermic? blank 2: is the reaction spontaneous or non-spontaneous?arrow_forward
- 8. The following constant pressure process occurs spontaneously when the system, initially at a different temperature from its surroundings, attains the same final temperature as the surroundings. Ar(g, 28 °C, 1 atm) ⟶ Ar(g, 70 °C, 1 atm) Which of the following statements about this process is/are correct? You may choose more than one, or none, of the statements. The entropy of the system increases: ΔS > 0. The entropy of the surroundings increases: ΔSsurr > 0. The entropy of the universe increases: ΔSuniv > 0. Work is done by the system on the surroundings. Heat flows from the system into the surroundings.arrow_forwardΣ *00 For a particular reaction, AH = 81.95 kJ and AS = 27.0 J/K. Calculate AG for this reaction at 298 K. AG = %3D What can be said about the spontaneity of the reaction at 298 K? O The system is spontaneous in the reverse direction. O The system is at equilibrium. O The system is spontaneous as written. 8:12 PH (口 四< 11/8/20 F5 F8 F12 PrtScr Insert Delete Backspace $4 % 8. 5. 7. 24 Alt Ctriarrow_forwardDoes the entropy increase or decrease in these reactions? Why? a) 4 Al + 3 O2 → 2 Al2O3 b) C + 2 Cl2 → CCl4 c) H2O (l) → H2O (g) d) CCl4 → C + 2 Cl2arrow_forward
- Calculate the standard enthalpy change for the reaction at 25 °C. Standard enthalpy of formation values can be found in this list of thermodynamic properties. MgCl₂ (s) + H₂O(1) AH;xn= V → B MgO(s) + 2 HCl(g) MacBook Air H N M 1 9 DD $10 P m kJarrow_forwardCalculate the standard entropy, Δ?∘rxn,ΔSrxn∘, of the reaction at 25.0 ∘C25.0 ∘C using the table of thermodynamic properties. 3C2H2(g)⟶C6H6(l) Δ?∘rxn= kJ⋅mol−1. Calculate the standard Gibbs free energy of the reaction, Δ?∘rxn. The standard enthalpy of the reaction, Δ?∘rxn, is −633.1 kJ⋅mol−1. Δ?∘rxn= kJ⋅mol−1 Determine in which direction the reaction is spontaneous as written at 25.0 ∘C25.0 ∘C and standard pressure. forward reverse both neitherarrow_forwarddetermine whether each reaction is spontaneous under standard conditions. If a reaction is not spontaneous, write the corresponding spontaneous reaction. K2O2 (s) → 2K (s) + O2(g) PbCO3 (s) → PbO (s) + CO2 (g) P4 (s) + 6H2(g) → 4PH3 (g) 2AgCl (s) + H2S (g) → Ag2S (s) + 2HCl (g)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY