
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
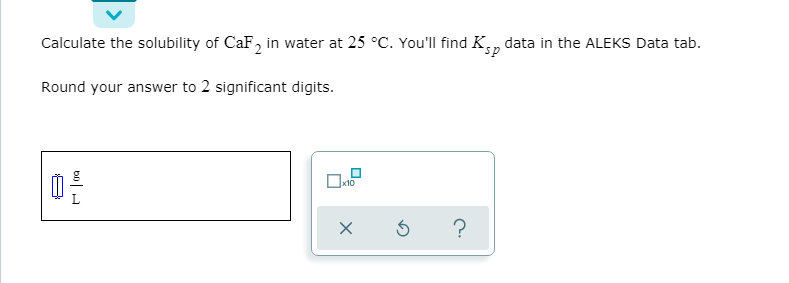
Transcribed Image Text:Calculate the solubility of CaF, in water at 25 °C. You'll find Kn
data in the ALEKS Data tab.
Round your answer to 2 significant digits.
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Similar questions
- Give detailed Solutionarrow_forward1. Listed below are solubility-vs.-temperature data for an organic substance A dis- solved in water. Temperature (°C) Solubility of A in 100 mL of Water (g) 1.5 20 3.0 40 6.5 60 11.0 80 17.0 a. Graph the solubility of A vs. temperature. Use the data given in the table. Connect the data points with a smooth curve. b. Suppose 0.1 g of A and 1.0 mL of water were mixed and heated to 80°C. Would all of substance A dissolve? c. The solution prepared in (b) is cooled. At what temperature will crystals of А appear? d. Suppose the cooling described in (c) were continued to 0°C. How many grams of A would come out of solution? Explain how you obtained your answer.arrow_forwardThe solubility of Fe(OH)2 in water at 25 °C is measured to be 5.2 × 10¯ Round your answer to 2 significant digits. 4 g 2. Use this information to calculate Ksp for Fe(OH)2. 日 ☐ x10 ☑arrow_forward
- Rearrange equation 1 to solve for the molecular weight of a compound assuming you know the mass of solute addedarrow_forwardCalculate the solubility of nitrogen gas in solution if the pressure of the gas mixture above the liquid is 1.53 atm. The gas mixture is 68.3% nitrogen, 20.1% oxygen and 11.6% carbon dioxide. -4 (KH for nitrogen is 6.1 × 10*M/ atm) 6.4 x 10M -4 а. -4 b. 9.3 x 10*M с. 6.1 x 10M d. 1.4x 10Marrow_forwardThe solubilities of three salts in water are shown in the graph. For each salt, determine how much will remain undissolved if 500 g is mixed into a liter of pure water at 40 °C. One significant figure is sufficient in each answer. Pb(NO3)2: KC1: K₂Cr₂O7: OD g 6.D Solubility (g/L) 1000 900 800 700 600 500 400 300 200 100 Pb(NO3)2 K₂Cr₂O, КСІ 0 10 20 30 40 50 60 70 80 90 100 Temperature (℃)arrow_forward
- BaCro4 according to aleks is 1.17 x 10^-10.arrow_forwardThe normal freezing point of a certain liquid X is 0.10 °C, but when 18. g of potassium bromide (KBr) are dissolved in 400. g of X the solution freezes at -1.2 °C instead. Use this information to calculate the molal freezing point depression constant K, of X. Round your answer to 2 significant digits. K₁ = 1 °C kg mol 1 Darrow_forwardhow is the solubility of a solid in a liquid affected by temperature?arrow_forward
- 178. Subject :- Chemistryarrow_forwardThe solubility in acetone of organic compound O is measured and found to be 0.762 that would contain 38.0 g of O at this temperature. Be sure your answer has the correct unit symbol and 3 significant digits. 0 x10 0.0 X μ 010 at 25. °C. Calculate the volume of a saturated solution of O in acetone mLarrow_forwardAt 20 °C and a partial pressure of 760 mmHg, the solubility of CO₂ in water is 0.169 g/100 mL. Part A What is the solubility of CO₂ at 2.0×104 mmHg? Express your answer using two significant figures. xa || ΑΣΦ Xb C= 3.38.10³ √x xx x x IXI Submit Previous Answers Request Answer * Incorrect; Try Again; 5 attempts remaining X.10 g/100 mLarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY