
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
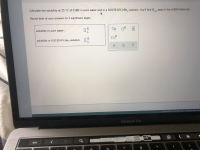
Transcribed Image Text:Calculate the solubility at 25 °C of CuBr in pure water and in a 0.0150 M CoBr , solution. You'll find K., data in the ALEKS Data tab.
Round both of your answers to 2 significant digits.
g
solubility in pure water:
x10
solubility in 0.0150 M CoBr2 solution: -
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the solubility at 25 °C of Ni(OH), In pure water and In a 0.0040M NaOH solutlon. You'll find Kn data In the ALEKS Data tab. 2. sp Round both of your answers to 2 significant digits. solubility In pure water: 日 x10 solubility in 0.0040 M N2OH solution: Check © 2021 McGraw-Hill Education. All Rights Reserve Explanationarrow_forwardPlease don't provide handwritten solution ....arrow_forwardO KINETICS AND EQUILIBRIUM Calculating the solubility of an ionic compound when a... Calculate the solubility at 25 °c of BaCro in pure water and in a 0.0170M BaCl₂ solution. You'll find ê data in the ALEKS Data tab. sp Round both of your answers to 2 significant digits. solubility in pure water: 17/0 solubility in 0.0170 M BaCl₂ solution: 0²/ 0 x10 X □ × ロ 0/3 8arrow_forward
- Calculate the solubility at 25 °C of BaCrO4 in pure water and in a 0.0170M BaCl₂ solution. You'll find data in the ALEKS Data tab. sp Round both of your answers to 2 significant digits. solubility in pure water: 0² 09 solubility in 0.0170 M BaCl₂ solution:: 0.2 X OxO 00 Śarrow_forwardCalculate the solubility at 25 °C of BaCrO, In pure water and in a 0.0110M BaCl, solution. You'll find K data In the ALEKS Data tab. 4 sp Round both of your answers to 2 significant digits. solubility in pure water: x10 solubility in 0.0110 M BaCl, solution: L Explanation Check Accessibility © 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy hp -> esc 23 $ 9- ! 5 6 3. 4 00arrow_forwardsolution. Calculate the solubility at 25 °C of Zn (OH), in pure water and in a 0.0030M ZNSO Round both of your answers to 2 significant digits. solubility in pure water: solubility in 0.0030 M ZnSO, solution:arrow_forward
- Ksp value for CaF2 is 3.45x10^-11arrow_forwardCalculate the solubility at 25°C of CuBr in pure water and in a 0.0060M CoBr2 solution. You'll find Ksp data in the ALEKS Data tab. Round both of your answers to 2 significant digits.arrow_forwardCalculate the solubility at 25 °C of PbCO, in pure water and in a 0.0130M Pb (NO₂), solution. You'll find K, data in the ALEKS Data tab. Round both of your answers to 2 significant digits. solubility in pure water: solubility in 0.0130 M Pb(NO₂) solution: 20 0²- X OxO 8 G dlo Solubility product constants (Ksp) Hg₂(CN)2 H9₂/2 HgS Hg₂SO4 PbBr₂ PbClz PbCO3 PbCrO4 PbF₂ pbl₂ Pb(10₂)2 www.a 5.00x10-40 5.2x10-29 4.00×10-54 6.5x10-7 6.60x10-5 1.70x10-5 7.40x10-14 2.00x 10-14 3.3x10-8 9.8×10-9 3.69x10-13. 78arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY