
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Calculate the root mean square speed of helium atoms and nitrogen molecules in m/s at 25°C
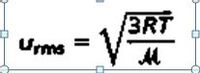
Transcribed Image Text:3RT
Urms
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Suppose that a woman weighing 130 Ib and wearing high heeled shoes momentarily places all her weight on the heel of one foot. If the area of the heel is 0.50 in.^2, calculate the pressure exerted on the underlying surface in (a) kilopascals, (b) atmospheres, and (c) pounds per square inch.arrow_forwardUsing the van der Waals equation, calculate the pressure of 2.94 moles of carbon tetrachloride (CCl4) gas held to a volume of 2.29 L and at a temperature of 565.8 K. (3 sf) =arrow_forwardUse the van der Waals equation to calculate the pressure (atm) exerted by 1.57 mol Cl_{2}2(g) confined to a volume of 1.50 L at 273K. For Cl_{2}2, a = 6.49 L^{2}2atm mol^{-2}−2, b = 0.0562 L mol^{-1}−1.arrow_forward
- What is the temperature (in K) of a sample of nitrogen with an root-mean-square velocity of 612.0 m/s The universal gas constant, R=8.3145 J/mol・K.?arrow_forwardMethane CH4 is a gas at room temperature. What is its root mean square velocityarrow_forwardDetermine the ratios of (i) the mean speeds, (ii) the mean translational kinetic energies of H2 molecules and Hg atoms at 20 °C.arrow_forward
- Determine the root mean square velocity of oxygen gas (in m/s) at a temperature of 190.2 °C.arrow_forwardThe partial pressure of the hydrogen gas collected is 0.928 bar. If the total volume of gas collected is 726 mL , what mass of hydrogen gas is collected? The molar mass of hydrogen gas is 2.016 g · mol Express your answer in grams to three significant figures. Πνα ΑΣφ MH2 garrow_forwardThe diameter of a Ne atom is 140 pm. What is the mean free path in nm of 0.5 mol Ne confined in 5 L at 300 K and 9.87 x 104 Pa?arrow_forward
- What is the temperature (in K) of a sample of helium with an root-mean-square velocity of 360.0 m/s? The universal gas constant, R=8.3145 J/mol・K.arrow_forwardAt what temperature will Ar atoms have the same root–mean–square speed, vRMS, as Ne atoms at 300 K? 600 K, 150 K, or 75 K Under the same conditions of temperature and pressure, if a volume of Ne gas effuses through a barrier in 10.0 min, an equal volume of which gas effuses through the same barrier in 4.5 min? Ar, H2, or Hearrow_forwardCalculate the root mean square (rms) average speed of the atoms in a sample of argon gas at 0.20 atm and -73. °C. Round your answer to 3 significant digits. E- 0- X [HO alharrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY