
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
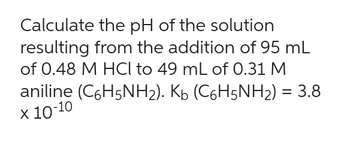
Transcribed Image Text:Calculate the pH of the solution
resulting from the addition of 95 mL
of 0.48 M HCl to 49 mL of 0.31 M
aniline (C6H5NH₂). K₁ (C6H5NH₂) = 3.8
x 10-10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What volume, in milliliters, of 0.180 M NaOH should be added to a 0.140 L solution of 0.029 M glycine hydrochloride (p Kal = 2.350, pK₁2 = 9.778) to adjust the pH to 2.50? NaOH volume = mLarrow_forward7.7 g of citric acid (MM = 192.1 g/mol) can be titrated with NaOH according to the following balanced chemical equation. H3C6H5O7 (aq) + NaOH(aq) → H2O(l) + Na H2C6H5O7(aq) what would the curve for the titration of the neutralization of the three hydrogens of this acid with NaOH look like?arrow_forwardHelp neededarrow_forward
- What is the pH of a saturated solution of Al(OH)3 at 25 oC, given that Ksp= 8.09 x 10-24 at this temperature?arrow_forwardRainwater is slightly acidic due to dissolved CO₂. Use the following data to calculate the pH of unpolluted rainwater at 22.6°C: vol % in air of CO₂ = 0.063 vol%; solubility of CO2 in pure water at 22.6°C and 1.09 atm is 79.29 mL CO2 per 100 mL, K₁1 of H₂CO3 = 5.27x10-7. Express the pH with 2 decimals. You might have to solve a quadratic.arrow_forwardSuppose a 0.040M aqueous solution of oxalic acid (H2C2O4) is prepared. Calculate the equilibrium molarity of C2O4 2−. You'll find information on the properties of oxalic acid in the ALEKS Data resource. Round your answer to 2 significant digits.arrow_forward
- Calculate the molar concentration of OH− ions in a 0.077 M solution of ethylamine (C2H5NH2; Kb=6.4x10-4arrow_forwardWhen a 0.013 M aqueous solution of a certain acid is prepared, the acid is 0.73% dissociated. Calculate the acid dissociation constant K of the acid. Round your answer to 2 significant digits. x = 0 x10 Xarrow_forwardCalculate the volume, in milliliters, of 0.170 M NaOH that must be added to 247 mL 0.0581 M 3‑(N‑Morpholino) propanesulfonic acid (MOPS) to give the solution a pH of 7.55. The pKa of MOPS is 7.18.arrow_forward
- In the titration of 25.00 mL of a water sample, it took 20.590 mL of 3.150x 10−3 M EDTA solution to reach the endpoint. The total hardness is due to one or a combination of Ca2+, Mg2+, and Fe2+ in your sample. It is convenient to express this hardness as though it was entirely due to Ca2+. Making this assumption, determine the number of moles of Ca2+ present in the bottled water sample titrated. (enter your answer with 3 significant figures)arrow_forwardWhat are the concentrations of H+ and OH− in asolution of 0.02 M NaOH?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY