
ENGR.ECONOMIC ANALYSIS
14th Edition
ISBN: 9780190931919
Author: NEWNAN
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
please answer in text form and in proper format answer with must explanation , calculation for each part and steps clearly
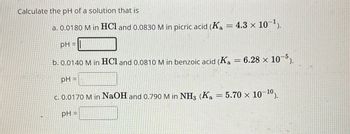
Transcribed Image Text:Calculate the pH of a solution that is
-
a. 0.0180 M in HCl and 0.0830 M in picric acid (Ka = 4.3 x 10¹).
pH=
b. 0.0140 M in HCl and 0.0810 M in benzoic acid (Ka = 6.28 × 105).
pH =
c. 0.0170 M in NaOH and 0.790 M in NH3 (Ka = 5.70 x 10-10).
pH=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- riginal price 3. Five years ago, Mary opened a savings account for a condo on the beach that earns 2.6% simple interest each year. She started the account with $550 and has not touched the account since. How much money is in the account now?arrow_forward3. If we deposit $5000 in an account that pays 9% per year. Find the amount of simple interest earned during 8 months. a)$3000 b)$300 c)$360 d)$3600arrow_forwardYou can earn 10% a year on your savings. Your dad offers you a Holiday Gift of $1000 this year, $2000 next Holiday, $3000 Holiday 2021. If instead he offered you $6,000 Holiday 2020 (next year). Which should you pick, the single payment or the 3 payments. SHOW YOUR WORK Note:- Do not provide handwritten solution. Maintain accuracy and quality in your answer. Take care of plagiarism. Answer completely. You will get up vote for sure.arrow_forward
- Exercise: 01 Issue a promissory note: ⑴Amount £3,026.00 ⑵Date and place of issue 8/August/2009,Guangzhou, China ⑶Tenor At 90 days after date ⑷Maker Guangdong Imp. & Exp. Co., Guangzhou ⑸Payee Chemicals Import & Export Company London ⑴Drawer Thames Enterprises Ltd., London ⑵Drawee The National Westminster Bank Ltd., London ⑶Payee Philips Hong Kong ⑷Date and place of issue 07/01/2001,London ⑸Amount GBP79,014 Exercise: 02 Issue a check:arrow_forwardTyped plz and asap thanksarrow_forwardAutoSave File Document! Word Chris Navo Home Insert Draw Design Layout References Mailings Review View MathType Help Acrobat Graphs Format Cobb and Douglas used economic data published by the government to obtain Table 2. Year P Year 1899 100 100 DOL 1911 148 216 1900 101 105 107 1912 155 1901 112 110 114 1912 1902 122 117 122 1014 169 152 244 1903 124 122 131 1915 109 156 266 1904 122 121 138 1916 225 183 1905 143 125 149 1917 227 1905 152 124 163 1915 223 201 1907 151 140 170 1919 218 19.08 126 123 485 1920 231 104 407 19.09 155 143 198 1921 179 146 417 1910 159 208 1922 240 161 431 Table 2 Swords et Predictions. The Cobb Douglass formula is P(L, K) = bLa K¹-a Determine monetary value of all the goods produced in 1 year or simply the production level in 1920 for a=.20 and b=1.01. Round to one decimal place.arrow_forward
- Typed plz Please provide me a solution stp by step i want quality solution also tske care of plagiarism alsoarrow_forwardThe case of transaction exposure is described as follows. Choose the correct answer in the parenthesis in the description of the case. The choice is in bold letters. My company sold a product to a German co., and sales department signed a contract to receive Euro 10,000 3 month later, A/R of Euro 10,000. The sales contract is delivered to my desk. I, a treasurer, am wondering how much USD my co. will get from Euro 10,000 3 months from now. The currency market is in turmoil, I will get more USD when USD weakens against EUR in the next 3 months. Market anticipates strong USD. However, if USD strengthens more than market expects, I will get (more /less) USD from sales contract of EUR 10,000. Are there any possibility to lock in my USD receipt to a certain level, or at minimum. In that way, my USD receipt will not get any lower even though USD appreciates more than the rate I can contract (buy EURO/sell EURO) now. How and what kind of contracts are available now to protect/hedge…arrow_forwardGiven the following information for an insurance company that writes 24-month term policies: Number of Vehicles 50 100 Policy Group A B Effective Date January 1, 2010 Expiration Date December 31, 2011 July 1, 2010 June 30, 2012 All policies within each group have the same effective date. (a) Calculate the earned car-years for calendar year 2011. (b) Calculate the earned car-years for policy year 2010 evaluated as of December 31, 2010 and as of December 31, 2011. (c) Assume Policy Group B cancels on January 1, 2011. Calculate the 2010 policy year written car- years evaluated as of December 31, 2010 and as of December 31, 2011 for Policy Group B. (d) Assume Policy Group B cancels on July 1, 2011. Calculate the 2010 and 2011 calendar year written car-years for Policy Group B.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Principles of Economics (12th Edition)EconomicsISBN:9780134078779Author:Karl E. Case, Ray C. Fair, Sharon E. OsterPublisher:PEARSON
Principles of Economics (12th Edition)EconomicsISBN:9780134078779Author:Karl E. Case, Ray C. Fair, Sharon E. OsterPublisher:PEARSON Engineering Economy (17th Edition)EconomicsISBN:9780134870069Author:William G. Sullivan, Elin M. Wicks, C. Patrick KoellingPublisher:PEARSON
Engineering Economy (17th Edition)EconomicsISBN:9780134870069Author:William G. Sullivan, Elin M. Wicks, C. Patrick KoellingPublisher:PEARSON Principles of Economics (MindTap Course List)EconomicsISBN:9781305585126Author:N. Gregory MankiwPublisher:Cengage Learning
Principles of Economics (MindTap Course List)EconomicsISBN:9781305585126Author:N. Gregory MankiwPublisher:Cengage Learning Managerial Economics: A Problem Solving ApproachEconomicsISBN:9781337106665Author:Luke M. Froeb, Brian T. McCann, Michael R. Ward, Mike ShorPublisher:Cengage Learning
Managerial Economics: A Problem Solving ApproachEconomicsISBN:9781337106665Author:Luke M. Froeb, Brian T. McCann, Michael R. Ward, Mike ShorPublisher:Cengage Learning Managerial Economics & Business Strategy (Mcgraw-...EconomicsISBN:9781259290619Author:Michael Baye, Jeff PrincePublisher:McGraw-Hill Education
Managerial Economics & Business Strategy (Mcgraw-...EconomicsISBN:9781259290619Author:Michael Baye, Jeff PrincePublisher:McGraw-Hill Education


Principles of Economics (12th Edition)
Economics
ISBN:9780134078779
Author:Karl E. Case, Ray C. Fair, Sharon E. Oster
Publisher:PEARSON

Engineering Economy (17th Edition)
Economics
ISBN:9780134870069
Author:William G. Sullivan, Elin M. Wicks, C. Patrick Koelling
Publisher:PEARSON

Principles of Economics (MindTap Course List)
Economics
ISBN:9781305585126
Author:N. Gregory Mankiw
Publisher:Cengage Learning

Managerial Economics: A Problem Solving Approach
Economics
ISBN:9781337106665
Author:Luke M. Froeb, Brian T. McCann, Michael R. Ward, Mike Shor
Publisher:Cengage Learning

Managerial Economics & Business Strategy (Mcgraw-...
Economics
ISBN:9781259290619
Author:Michael Baye, Jeff Prince
Publisher:McGraw-Hill Education