
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please answer all questions I'll give you like upvote
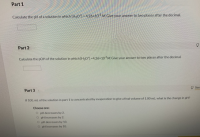
Transcribed Image Text:Calculate the pH of a solution in which (HO) = 4.26×10 M. Give your answer to two places after the decimal.
Part 2
Calculate the pOH of the solution in which (HO)-4.26×10M Give your answer to two places after the decimal.
Part 3
If 100. ml. of the solution in part 1 is concentrated by evaporation to give a final volume of 1.00 ml, what is the change in pH?
Choose one:
O pH decreases by 2.
O pH increases by 2.
O pH decreases by 10.
O pH increases by 10.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Two strategies are also followed when solving for the pH of a base in water. What is the strategy for calculating the pH of a strong base in water? List the strong bases mentioned in the text that should be committed to memory. Why is calculating the pH of Ca(OH)2 solutions a little more difficult than calculating the pH of NaOH solutions? Most bases are weak bases. The presence of what element most commonly results in basic properties for an organic compound? What is present on this element in compounds that allows it to accept a proton? Table 13-3 and Appendix 5 of the text list Kb values for some weak bases. What strategy is used to solve for the pH of a weak base in water? What assumptions are made when solving for the pH of weak base solutions? If the 5% rule fails, how do you calculate the pH of a weak base in water?arrow_forward(a) What is the pH of a 0.105 M HCl solution? (b) What is the hydronium ion concentration in a solution with a pH of 2.56? Is the solution acidic or basic? (c) A solution has a pH of 9.67. What is the hydronium ion concentration in the solution? Is the solution acidic or basic? (d) A 10.0-mL sample of 2.56 M HCl is diluted with water to 250. mL What is the pH of the dilute solution?arrow_forwardFind [OH+], [OH-] and the pH of the following solutions. (a) 30.0 mL of a 0.216 M solution of HCI diluted with enough water to make 125 mL of solution. (b) A solution made by dissolving 275 mL of HBr gas at 25C and 1.00 atm in enough water to make 475 mL of solution. Assume that all the HBr dissolves in water.arrow_forward
- Calculate [OH-] and pH in a solution in which the hydrogen sulfite ion, HSO3-, is 0.429 M and the sulfite ion is (a) 0.0249 M (b) 0.247 M (c) 0.504 M (d) 0.811 M (e) 1.223 Marrow_forwardA 10.0-mL sample of an HCl solution has a pH of 2.000. What volume of water must be added to change the pH to 4.000?arrow_forward12.62 Write the formula of the conjugate acid of each of the following bases, (a) OH-, (b) NHj, (c) CHjNHt, (d) HPO/-, (e) CO.,2’arrow_forward
- For oxyacids, how does acid strength depend on a. the strength of the bond to the acidic hydrogen atom? b. the electronegativity of the element bonded to the oxygen atom that bears the acidic hydrogen? c. the number of oxygen atoms? How does the strength of a conjugate base depend on these factors? What type of solution forms when a nonmetal oxide dissolves in water? Give an example of such an oxide. What type of solution forms when a metal oxide dissolves in water? Give an example of such an oxide.arrow_forwardWhat is a salt? List some anions that behave as weak bases in water. List some anions that have no basic properties in water. List some cations that behave as weak acids in water. List some cations that have no acidic properties in water. Using these lists, give some formulas for salts that have only weak base properties in water. What strategy would you use to solve for the pH of these basic salt solutions? Identify some salts that have only weak acid properties in water. What strategy would you use to solve for the pH of these acidic salt solutions? Identify some salts that have no acidic or basic properties in water (produce neutral solutions). When a salt contains both a weak acid ion and a weak base ion, how do you predict whether the solution pH is acidic, basic, or neutral?arrow_forwardTo measure the relative strengths of bases stronger than OH, it is necessary to choose a solvent that is a weaker acid than water. One such solvent is liquid ammonia. (a) Write a chemical equation for the autoionization of ammonia. (b) What is the strongest acid and base that can exist in liquid ammonia? (c) Will a solution of HCI in liquid ammonia be a strong electrical conductor, a weak conductor, or a nonconductor? (d) Oxide ion (O2) is a stronger base than the amide ion (NH2). Write an equation for the reaction of O2 with NH3 in liquid ammonia. Will the equilibrium favor products or reactants?arrow_forward
- Students are often surprised to learn that organic acids, such as acetic acid, contain OH groups. Actually, all oxyacids contain hydroxyl groups. Sulfuric acid, usually written as H2SO4, has the structural formula SO2(OH)2, where S is the central atom. Identify the acids whose structural formulas are shown below. Why do they behave as acids, while NaOH and KOH are bases? a. SO(OH)2 b. ClO2(OH) c. HPO(OH)2arrow_forwardThe pH of 1.0 108 M hydrochloric acid is not 8.00. The correct pH can be calculated by considering the relationship between the molarities of the three principal ions in the solution (H+, Cl, and OH). These molarities can be calculated from algebraic equations that can be derived from the considerations given below. a. The solution is electrically neutral. b. The hydrochloric acid can be assumed to be 100% ionized. c. The product of the molarities of the hydronium ions and the hydroxide ions must equal Kw. Calculate the pH of a 1.0 108-M HCl solution.arrow_forwardAre solutions of the following salts acidic, basic, or neutral? For those that are not neutral, write balanced equations for the reactions causing the solution to be acidic or basic. The relevant Ka, and Kb values are found in Tables 13-2 and 13-3. a. Sr(NO3)2 b. NH4C2H3O2 c. CH3NH3Cl d. C6H5NH3ClO2 e. NH4F f. CH3NH3CNarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning