
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
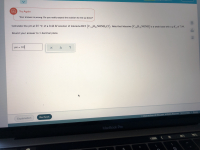
Transcribed Image Text:Calculate the pH at 25 °C of a 0.44 M solution of lidocaine HCI (C14H2NONH,CI). Note that lidocaine (C14H2NONH) is a weak base with a p Kb of 7.94.
Round your answer to 1 decimal place.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the following acid-base equilibria of weak acids in water, label the acid (A), the base (B), the conjugate acid (CA), and the conjugate base (CB). HCIO,(aq) + H,O(1) = H,O*(aq) + C1O, (aq) H₂CO3(aq) + H₂O(1) H₂O+ (aq) + HCO3(aq) H,O(1) +CH,NH (aq) = CH,NH,(aq) + H,O*(aq) O CH₂COOH(aq) + H₂O(1) ⇒ CH₂COO¯(aq) + H₂O+ (aq) O B Answer Bank CA A CBarrow_forward0.14 M CH3NH2 (where K for CH3NH2 is 4.4 x 10-4) Express your answer using two decimal places. pH = 阿 ΑΣΦ ?arrow_forwardDetermine the pH of a weak acid, HA, that has a concentration of 0.015 M and a Ka = 0.00005. Enter your answer to two places after the decimal.arrow_forward
- Based on the data in the table below, which of the following is the strongest base? Organic Acid Formic acid (HCOOH) K, pK, 1.8 X 10 4 3.74 Acetic acid (CH;COOH) 1.8 X 105 4.74 Propanoic acid (CH3CH2COOH) Ascorbic acid (vitamin C) 1.3 X 10 5 4.89 7.9 X 10 5 4.10 OCH;COONA O The conjugate base of ascorbic acid OHCOONa O CH;CH2COONAarrow_forwardCalculate the pH at 25 °C of a 0.62 M solution of lidocaine HCI (C₁4H₂NONH₂CI). Note that lidocaine (C₁4H₂NONH) is a weak base with a pk of 7.94. Round your answer to 1 decimal place.arrow_forwardWhat is the partial pressure of carbon dioxide in a container that holds 5 moles of carbon dioxide, 3 moles of nitrogen, and 1 mole of hydrogen and has a total pressure of 1.05atm? Show the complete solution. a. PCO2 = 1.588atm; PN = 1.347atm; PH = 1.116atm b. PCO2 = 0.588atm; PN = 0.347atm; PH = 0.116atm c. PCO2 = 2.588atm; PN = 2.347atm; PH = 2.116atm d. PCO2 = 3.588atm; PN = 3.347atm; PH = 3.116atmarrow_forward
- Glutamine (HQ) is a diprotic amino acid with K₁1= 6.5 x 10-3 and K₁2 = 1.00 x 10. Determine the pH of each of the solutions. A 0.276 M glutamine hydrochloride (H₂Q*CI) solution. pH = 1.373088781 Incorrectarrow_forwardThe acid dissociation constant K, of hypobromous acid (HBRO) is 2.3 x 10 D. Calculate the pH of a 3.3 M solution of hypobromous acid. Round your answer to 1 decimal place. pH = %3D Continuearrow_forwardConsider the following data on some weak acids and weak bases: acid base Ka name formula name formula HCH3CO2 1.8 × 10 C,H;NH, 4.3 × 10 5 10 acetic acid aniline hydrofluoric acid HF 6.8 x 10 ethylamine C2H;NH2| 6.4 x 10 Use this data to rank the following solutions in order of increasing pH. In other words, select a 'l' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution pH 0.1 М KF choose one 0.1 M C2H5NH3CI choose one 0.1 M NaCl choose one 0.1 M NaCH3CO2 choose onearrow_forward
- The pH scale depends on the value of Kw. What would the pH of neutral water be if the Kw was 5.54 x10-15? IMPORTANT: When entering your answer: •Enter the number only (no units) • Do not leave any spaces Use a leading zero before the decimal when necessary Report your number to 3 decimal places (regardless of the significant figures) • Correctly round your answer to the 3rd decimal place (allowed margin of error is only ± 0.001, so always use un- rounded numbers in your calculations) Examples: 0.123 or 9.876arrow_forwardCalculate the pH at 25 °C of a 0.16M solution of lidocaine HCl (C14H2, NONH2C1). Note that lidocaine (C14H21 NONH) PK of 7.94. Round your answer to 1 decimal place. pH = 0 is a weak base with aarrow_forwardComplete the table below. Round each of your entries to 2 significant digits. You may assume the temperature is 25 °C. conjugate acid formula 0 0 ΗΝΟ, Ka 0 0 4.5 X 10 4 conjugate base Kb formula CH,NH, HCO3 П 4.4 X 10 2.2 × 10 0 4 8 90 X S x10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY