
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
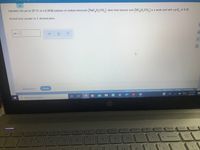
Transcribed Image Text:Calculate the pH at 25 °C of a 0.30M solution of sodium benzoate (NaC,H,CO,). Note that benzoic acid (HC,H,Co,) is a weak acid with a p K, of 4.20.
Round your answer to 1 decimal place.
PH =
de
Explanation
Check
O 2021 McGraw-Hil Education. All Rights Reserved. Tarms of Use f Privacy Accessiblity
5:13 PM
O Type here to search.
W
5/8/2021
40
144
prt so
delete
home
end
num
8.
6.
backspace
lock
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A solution is prepared at 25 °c that is initially 0.21M in nitrous acid (HNO,), a weak acid with K,=4.5 x 10 4, and 0.43M in potassium nitrite (KNO,). Calculate the pH of the solution. Round your answer to 2 decimal places. pH = ?arrow_forwardThank you!arrow_forward1 2 4 Calculate the pH at 25 °C of a 0.59M solution of sodium hypochlorite (NaClO) . Note that hypochlorous acid (HC10) is a weak acid with a p K, of 7.50. Round your answer to 1 decimal place. pH = Software update ready to install. Su Continue O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C II のarrow_forward
- A solution is prepared at 25 °C that is initially 0.16 M in benzoic acid (HC,H,CO,), a weak acid with K,=6.3 × 10 °, and 0.058 M in sodium benzoate -5 %3D (NaC H,CO,). Calculate the pH of the solution. Round your answer to 2 decimal places.arrow_forwardThe Kb for four bases are CN'- = 1.6 x 10-s, CO;²- = 2.1 x 104, NH3 = 1.8 x 10“, F'- = 1.5 × 10-11. If the pH's of 0.50 mol/L solutions of each of these weak bases were measured and they were placed in order from highest pH to lowest the result would be which of the following? a. CN', CO3", NH3, F'- b. CO;?,NH:, CN', F'- c. F', CN',NH3,CO;?- d. CO?²,CN',NH3, F'- е. none of the abovearrow_forward3. Lactic acid, C,H;O; (90), is a weak acid that gives yogurtits sour taste. Calculate the pH of a 0.0010 mol/L solution of lactic acid. The K, for lactic acid is 1.4 x 104 16 INQarrow_forward
- A solution is prepared at 25 °C that is initially 0.15M in propanoic acid (HC₂H-CO₂), a weak acid with K=1.3 × 105, and 0.18M in potassium propanoate (KC₂H,CO₂). Calculate the pH of the solution. Round your answer to 2 decimal places. pH = 0 X Sarrow_forwardFor each of the following entities • Identify it as a strong or weak acid. • Select the correct arrow to show the changes that occur when the acids are placed in water. • Select the approximate pH for each resulting solution. HCI(aq) is classified as a + acid. The reaction equation with water utilizes which of the following arrows? Choice A Choice B 7 >>7 H2SO3(aq) is classified as a The reaction equation with water would utilize which of the following arrows? Choice A Choice B < 50% H2SO3(aq) + H20(I) • HSO3 (aq) + H30*(aq) The approximate pH of the solution would bearrow_forwardCalculate the pH at 25 °C of a 0.51M solution of sodium benzoate (NaC,H,CO,). Note that benzoic acid (HC,H,CO,) is a weak acid with a p K, of 4.20. Round your answer to 1 decimal place. pH =||arrow_forward
- A solution is prepared at 25 °c that is initially 0.32M in propanoic acid (HC,H,CO,), a weak acid with K,=1.3 × 10, and 0.23 M in potassium propanoate (KC,H,CO,). Calculate the pH of the solution. Round your answer tO 2 decimal places. pHarrow_forwardA solution is prepared at 25 °C that is initially 0.27M in chlorous acid (HCI0,), 2 a weak acid with K,=1.1 × 10 and 0.29M in potassium chlorite (KCIO,). Calculate the pH of the solution. Round your answer to 2 decimal places. pH = U ? olo Ararrow_forwardSuppose you want to prepare a solution with a pH of 4.00 using acetic acid, CH3COOH, and sodium acetate, CH3CoOONa. What should the ratio of sodium acetate to acetic acid, CH3CO0-| : [CH3COOH], be in your buffer solution? Hint: The Ka for acetic acid is 1.8x105. 0.18:1 O 1.9:1 0.84:1 O 5.5:1 O 1:1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY