
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
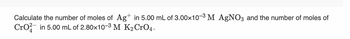
Transcribed Image Text:Calculate the number of moles of Ag+ in 5.00 mL of 3.00x10-3 M AgNO3 and the number of moles of
CrO2 in 5.00 mL of 2.80×10-3 M K₂ CrO4.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3) Determine the mass of solute in 3.60 L of 0.200 M acetic acid, HC,H3O2.arrow_forwardYou need to prepare 1.0 L of 2.0 M H2SO4. In the store room is a bottle of 18.0 M H2SO4. What volume of concentrated acid do you need to measure out and what volume of water do you need to add to prepare your solution?arrow_forwardHow many grams of K2SO4 are there in 33.6 ml of 2.56 M K2SO4 solution?arrow_forward
- In the reaction below, how many grams of chlorine gas (Cl₂) are needed to react with 5.393 x 1023 molecules of sodium hydroxide? Cl₂(g) + NaOH(aq) → NaCl(aq) + NaClO3(aq) + H₂O(l) What volume (in L) of 0.125 mol/L Ba(OH)2 solution is needed to react completely with 0.200 L of a 0.300 mol/L HNO3 solution according to the following reaction: Ba(OH)2(aq) + HNO3(aq) → Ba(NO3)2(aq) + H₂O(l) ->>arrow_forward2. Calculate the molarity of the sulfate ion if 0.46 g SO4 2 is present in 50.0 mL of solutionarrow_forwardHow many moles of precipitate will be formed when 50.0 mL of 0.300 M AgNO₃ is reacted with excess CaI₂ in the following chemical reaction? 2 AgNO₃ (aq) + CaI₂ (aq) → 2 AgI (s) + Ca(NO₃)₂ (aq)arrow_forward
- How many grams of Fe(NO3)3 must be used to prepare 1.50 L of a 0.0625 M Fe(NO3)3 solution?arrow_forwardHow many chloride ions are present in 65.5 mL of 0.210 M AICI3 solution? 4.02 x 1023 chloride ions 5.79 x 1024 chloride ions 2.48 x 1022 chloride ions 8.28 x 1021 chloride ions 1.21 x 1022 chloride ionsarrow_forwardWhich of the following solutions of strong electrolytes contains the largest number of ions? 400.0 mL of 0.100 M KI O 200.0 mL of 0.200 M CACI, O300.0 mL of 0.150 M Cs PO,arrow_forward
- MgCO3(s) + 2 HCl(aq) → MgCl2(aq) + CO2(g) + H2O(l) Calculate the volume of 0.440 M HCl needed for 12.8 g MgCO3 to react completely.arrow_forwardA student pipets 18 mL of a lithium sulfate solution into a 164 mL volumetric flask and dilutes to the mark. He analyzes the diluted solution and finds its concentration to be 0.0078 M NaNO3 . What was the concentration of the NaNO3 in the original solution?arrow_forwardA student pipets 12 mL of a lithium sulfate solution into a 163 mL volumetric flask and dilutes to the mark. He analyzes the diluted solution and finds its concentration to be 0.0072 M NANO3 . What was the concentration of the NaNO3 in the original solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY