
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
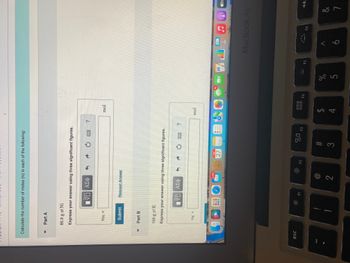
Transcribed Image Text:Calculate the number of moles (n) in each of the following:
esc
Part A
85.9 g of Ni
Express your answer using three significant figures.
INI
Submit
Part B
IVD ΑΣΦ
NK =
Request Answer
159 g of K
Express your answer using three significant figures.
LIVE ΑΣΦ
FI
5,386
@
2
F2
JLE
27
O
C
20
#3
F3
?
mol
mol
000
$
4
F4
%
сторо
F5
MacBook Air
F6
&
7
tv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- This is the chemical formula for methyl tert-butyl ether (the clean-fuel gasoline additive MTBE): CH₂OC (CH3)₂ A chemical engineer has determined by measurements that there are 31.9 moles of carbon in a sample of methyl tert-butyl ether. How many moles of hydrogen are in the sample? Round your answer to 3 significant digits. mol x10arrow_forwardHow many moles are in 11.50 g of CO₂? Show your work. H Normal ✿ ==== =A Enter your answer here DI G BIU √xarrow_forwardDUE NOW. Please answer it with the correct significant figures. Thank you!arrow_forward
- 6 CO2 (g) + 6 H20 (- CH1206 (s) + 6 O2 (g) How many grams of carbon dioxide would be required to produce 42.5 g C,H1206? In the 'Answer' box, express your numerical answer to the nearest ±0.1arrow_forwardO 00 5 Phosphorus reacts with oxygen to form diphosphorus pentoxide, P,O,. 4 P(s) + 50,(g) –→ 2 P,0,(s) How many grams of P,O, are formed when 6.71 g of phosphorus reacts with excess oxygen? Show the unit analysis used for the calculation by placing the correct components into the unit-factor slots. 6.71 g P x = g P,O, Answer Bank 32.00 g 0, 4 mol P I mol P 30.97 g P 2 mol P,O, I mol P,0, I mol O, 141.94 g P,0, 5 mol 0, grams of P,0,: g P,0,arrow_forward2. Early rockets used a fuel composed of hydrazine (N2H4) and dinitrogen tetraoxide (N204), which react on contact to form nitrogen gas and water vapor. How many grams of nitrogen gas form when 1.00 x102 g of N2H4 and 2.00 x102 g of N204 are mixed?arrow_forward
- Part A A mixture of N2(g) and H2(g) reacts in a closed container to form ammonia, NH3(g). The reaction ceases before either reactant has been totally consumed. At this stage 3.4 mol N2, 3.4 mol H2, and 3.4 mol NH3 are present. How many moles of N2 and H2 were present originally? Express your answer using two significant figures. Enter your answers numerically separated by a comma nN₂,nH₂ = Ο ΑΣΦ Submit Request Answer Provide Feedback MacBook Pro ? molarrow_forwardButyric acid, C4H8O2, is a compound found in many fats and oils. A 129.6 g sample of butyric acid is how many moles of butyric acid? Report the answer to 3 significant figures.arrow_forwardAnswer the following questions regarding this chemical reaction: Cu(s) + HNO3(aq) --> Cu(NO3)2(aq) + H2O(l) + NO2(g) a. If you have 32.5 g of Cu, how much Cu(NO3)2 is produced? Use the correct number of significant figures.arrow_forward
- Could you please answer these questions for me? They are all multiple parts of one long FRQ.arrow_forwardA high-performance heater that burns propane, C3H8(g), is adjusted so that 100.0 g of O2(g) enters the system for every 100.0 g of propane. Calculate the mol ratio of oxygen to propane. Write the answer as a decimal containing number. O2 : C3H8 Is this mixture rich or lean?arrow_forwardLiquid hexane CH3CH24CH3 reacts with gaseous oxygen gas O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O . If 38.8g of water is produced from the reaction of 48.26g of hexane and 260.5g of oxygen gas, calculate the percent yield of water. Round your answer to 3 significant figures.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY