
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
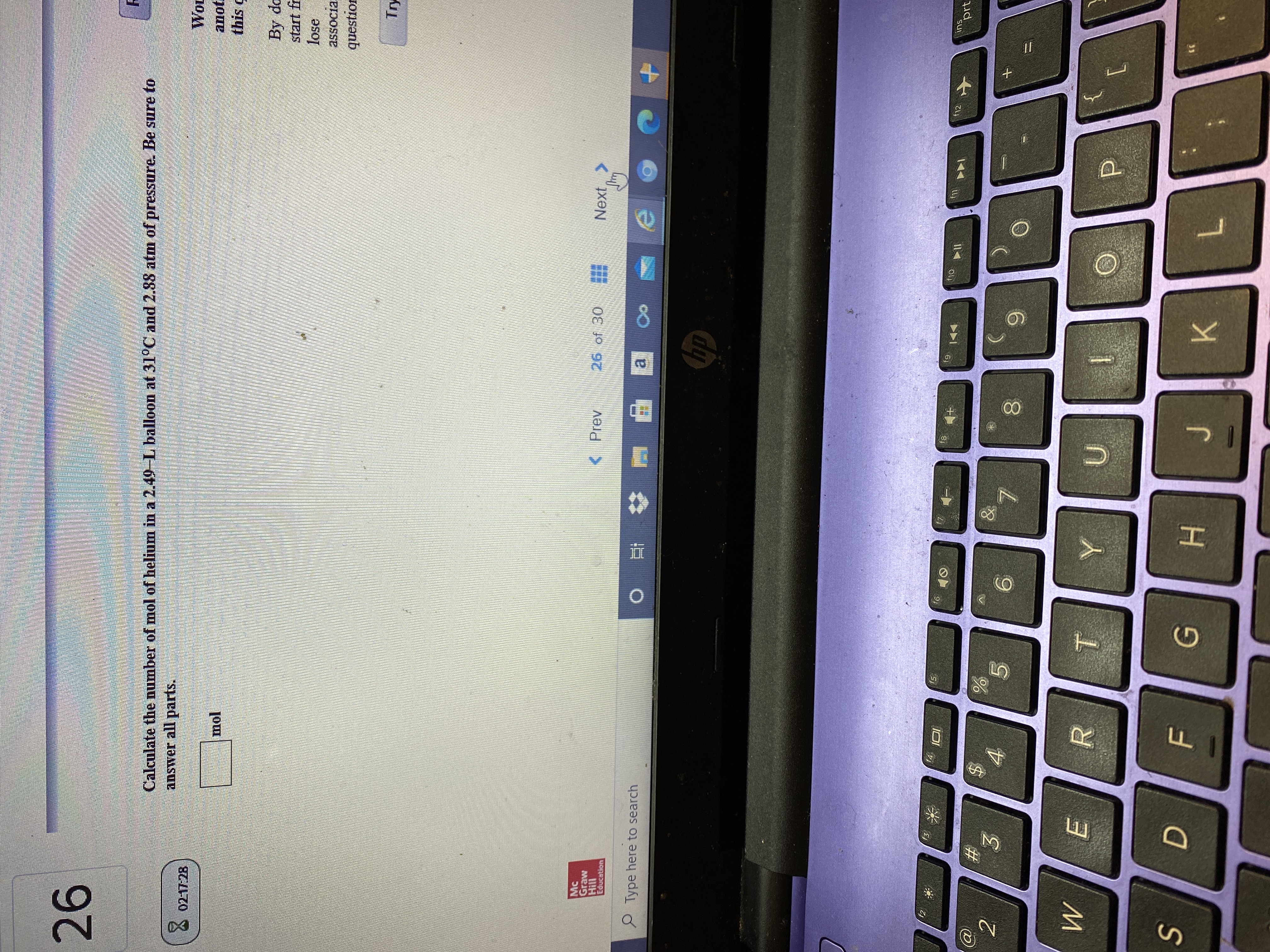
Transcribed Image Text:Calculate the number of mol of helium in a 2.49-L balloon at 31°C and 2.88 atm of pressure. Be sure to
answer all parts.
下
mol
ai
th
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 3.42 gram sample of an unknown gas is found to occupy a volume of 1.90 L at a pressure of 547 mm Hg and a temperature of 33 °C. The molecular weight of the unknown gas is ___ g/mol.arrow_forwardA rigid plastic container holds 42.13 g of propane gas (C3H8) at a pressure of 890.1 torr. What is the pressure (in torr) if 6.79 g of propane is removed at constant temperature? Enter to 1 decimal place.arrow_forwardA sample of gas occupies 748.7 mL, at 7.76 atm, and 152.4°C. How many moles are in the sample of gas? Do not put units in your answer. Record your answers to 2 decimal places.arrow_forward
- If a sample of helium gas has a volume of 120 mL and a pressure of 850 mm Hg, what is the new volume if the pressure is changed to 425 mm Hg (n and T are constant)?arrow_forwardA balloon is filled with 2 liters of air at sea level. If the balloon is taken up a mountain so that the new air pressure is approximately 0.94 atmospheres, what will the new volume of the balloon be? Round to the nearest hundredtharrow_forwardWhat mass of helium gas is required to fill a 5.0L balloon to a pressure of 1.1 atm at 25oC.arrow_forward
- A flask contains a mixture of He and Ne at a total pressure of 3.51 atm. There are 2.54 mol of He and 3.45 mol of Ne in the flask. The partial pressure of the He is atm. Give your answer to 3 significant digits.arrow_forwardConvert the pressure of 3.4 atm to mm Hg. Answer in units of mm Hg.arrow_forwardA mixture of neon and nitrogen gases is maintained in a 8.77 L flask at a pressure of 2.88 atm and a temperature of 82 °C. If the gas mixture contains 4.74 grams of neon, the number of grams of nitrogen in the mixture is ____ g.arrow_forward
- What volume is occupied by a 1.38 mol sample of helium gas at a temperature of 0 °C and a pressure of 1 atm?______Larrow_forwardA balloon of H2 and a balloon of O2 are floating in our classroom. Thus, they're at the same pressure and temperature. The volume of H2 balloon is 4 times the volume of the O2 balloon. What is ratio between the mass of H2 and the mass of O2? mass H2 over mass O2arrow_forwardFor questions 2-4 show all calculations. 2 A 100-milliliter sample of a gas at a pressure of 380. torr is reduced to 190. torr at con- stant temperature. What is the new volume of the gas?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY