
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:RB #
My Questions | bartleby
PB 11.4 Colligative Properties- Ch X
https://ezto.mheducation.com/ext/map/index.html?_con=con&external_brows
Saved
3 attempts left
Check my work
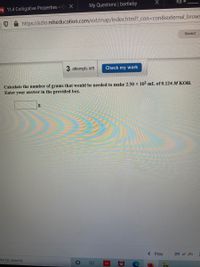
Calculate the number of grams that would be needed to make 2.50 × 10² mL of 0.124 M KOH.
Enter your answer in the provided box.
< Prev
20 of 25
ere to search
Expert Solution
arrow_forward
Step 1

Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the volume of 0.145 M NaOH solution required to neutralize each sample of hydrochloric acid. The neutralization reaction is:NaOH(aq)+HCl(aq)→H2O(l)+NaCl(aq) Part A 20 mL of a 0.145 M HCl solution Express your answer to two significant figures and include the appropriate units.arrow_forwardIn the laboratory you dissolve 14.9 g of barium acetate in a volumetric flask and add water to a total volume of 500 mL.What is the molarity of the solution? What is the concentration of the barium cation? What is the concentration of the acetate anion?arrow_forwardIn the acid-base neutralization reaction between 0.75 M H2SO4 (aq) and 0.75 M KOH (aq), the acid and base will react in a molar ratio of mole acid to mole base.arrow_forward
- Hydrochloric acid solution is commonly found on laboratory shelves at a concentration of 6.00 M. How many mL of this concentrated HCl would you dilute in order to prepare 500. mL of 1.24M HCl(aq)arrow_forwardHow many mL of 0.150 M Ca(OH)2(aq) will be required to completely react with 150. mL of 0. 100 M HCl(aq) solution. Express your answer in decimal notation.arrow_forwardConsider the balanced reaction below. FeCl3 (aq) + 3 NaOH (aq) --> Fe(OH)3 (s) + 3 NaCI (aq) A 0.532 M NaOH solution is added to 34.9 mL of a 0.398 M FeCl3 solution. What is the volume of NaOH in mL that must be added to completely react with the FeCl3 solution? Round your answer to three significant figures.arrow_forward
- On their third titration trial, the CHM 111 student used 18.80 mL of 0.205 M NaOH(aq) to neutralize 25.0 mL of HCl(aq). Calculate the molar concentration of the HCl solution to the correct number of significant figures.arrow_forwardA 25.0 mL sample of phosphoric acid requires 22.5 mL of 1.1 M sodium hydroxide for complete neutralization. What is the molarity of the phosphoric acid? The neutralization reaction : H3PO4 + 3NaOH ----> Na3PO4 + 3H2O Write your numerical answer without unit inside the box. The answer should be in Two Significant figures and in standard, not scientific notation.arrow_forwardA solution containing 405.6 mM CaCl₂ is combined with an equal volume of 176.1 mM NaCl. What is the concentration of chloride (CI) ions in the resulting solution? Express your answer using units of millimolar (mM) using at least three significant figures.arrow_forward
- A saline solution contains 0.770 g of NaCl (molar mass = 58.55 g/mol) in 118 mL of solution. The concentration is 0.112 M. What is the concentration of Na and Cl ions in this solution given in molarity and rounded to 3 significant figures?arrow_forwardA chemist prepares a solution of potassium bromide KBr by measuring out 0.17g of KBr into a 300.mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Br− anions in the chemist's solution. Be sure your answer is rounded to 2 significant digits.arrow_forwardSodium hydroxide is used extensively in acid-base titrations because it is a strong, inexpensive base. A sodium hydroxide solution was standardized by titrating 43.00 mL of 0.1425 M standard hydrochloric acid. The initial buret reading of the sodium hydroxide was 1.07 mL, and the final reading was 38.97 mL. What was the molarity of the base solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY