
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
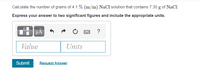
Transcribed Image Text:Calculate the number of grams of 4.1 % (m/m) NaCl solution that contains 7.30 g of NaCl.
Express your answer to two significant figures and include the appropriate units.
µA
Value
Units
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the quantity (mL) of glycerin needed to prepare 250 mL of the following ophthalmic solution. Report your answer to 2 decimal places. DO NOT include units. Erythromycin lactobionate Dexamethasone sodium phosphate Glycerin Sterile water for injection, ad 500 mg 100 mg 2.5 mL 100 mLarrow_forwardMacmillan Learning Chemistry: Fundamentals and Principles Davidson Calculate the volume percent of solute in each of the solutions. A solution made by adding 20.3 mL of methyl alcohol to enough water to give 467 mL of solution. % (v/v) = A solu presented by Macmillan Learning % (v/v) = made by adding 78.3 mL of ethylene glycol to enough water to give 173 mL of solution. % %arrow_forwardDetermine the mass of NaCl in grams needed to make the following solution: 4 % NaCl in a total volume of 126 mL Report your answer to the nearest tenth of a gramarrow_forward
- Calculate the volume in milliliters of 6.5 % (v/v) ethanol solution that contains 33.7 mL of ethanol. Express your answer to two significant figures and include the appropriate units. HÀ ? Value Units Submit Request Answerarrow_forwardWhat is the mass of sugar in 43.0 grams of a 1.05% sugar solution. Please show how to get answerarrow_forwardFind the percent by mass of sodium chloride in a 1.20 M NaCl solution. The density of the solution is 1.07 g/mL. [ΨΕΙ ΑΣΦ esc Submit Provide Feedback ! 1 Request Answer F1 A @ 2 F2 W S ? #3 80 F3 % E S4 $ D a F4 R % 5 F 2 F5 MacBook Air T 6 F6 G & < 7 Yarrow_forward
- Find grams of solvent in a 5.6% mass of solutions of X that has 22.3g(X) g(soltn)= g( )+g( ) --> g( ) = g( )–g( ) 5.6 % mass X means: ____g(X) ⇔ ____g(________) 22.3 g(X) × ______g(soltn)/________g(X) = ______g(soltn) --> _____g(soltn) – _____g(X)=______g(solv)arrow_forwardI Review I Constants I Periodic Table 0.30 mol of LİNO3 in 6.12 L of solution Express your answer using two significant figures. ΑΣφ ? molarity %D Submit Request Answer Part B 74.3 g C2 H6 O in 2.26 L of solution Express your answer using three significant figures. DA ΑΣφ molarity Submit Request Answer Part C 12.83 mg KI in 102.7 mL of solution Express your answer using four significant figures. DA ΑΣφ ?arrow_forwardWhat mass ( in g ) of NaCl is needed to make 590 g of a 11.0% NaCl solution? Report your answer to 2 decimal places. Your Answer: Answer unitsarrow_forward
- I need help with this question please! Use dimensional analysis please!?arrow_forwardQUESTION 3 Use the graph to estimate the solubility of sugar in 100 mL of water at 30 °C. Solubility of Sugar in Water Versus Temperature Solubility (g sugar/100 ml water) 0 0 0 0 500 450 400 350 300 250 200 150 100 150 g 200 g 215 g 255 g 0 20 10 Temperature (°C) 60 80 100arrow_forwardHow many grams of a solution whose % (m/m) = 21% contain 40.0 g of solute?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY