
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:Calculate the molarity of the solution when 14 mL of solution contains 0.128 g of ammonium phosphate.
Is a molar mass needed for this calculation? Enter either yes or no.
If so, calculate the molar mass using our periodic table and enter with the proper s.f. If not, enter 0. Do not include units.
g/mol
In the three blanks, enter the results of the calculation: value (decimal notation with proper s.f.), units, and substance (in this order).
Expert Solution
arrow_forward
Step 1
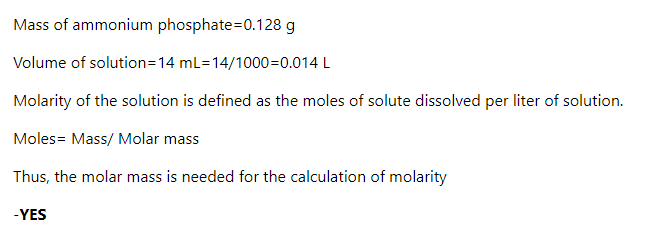
arrow_forward
Step 2
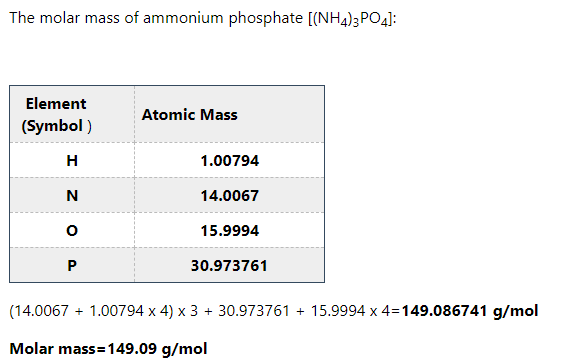
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A barium hydroxide solution is prepared by dissolving 3.22 g of Ba(OH), in water to make 58.1 mL of solution. What is the concentration of the solution in units of molarity? concentration: The barium hydroxide solution is used to titrate a perchloric acid solution of unknown concentration. Write a balanced chemica equation to represent the reaction between barium hydroxide and perchloric acid. chemical equation: If 18.5 ml of the barium hydroxide solution was needed to neutralize a 8.04 mL aliquot of the perchloric acid solution, what is the concentration of the acid? concentration:arrow_forward4.67 g of cobalt(II) sulfate are dissolved in water to make 2600. mL of solution. Calculate the molarity. Isa molar mass needed for this calculation? Enter either yes or no, If so, calculate the molar mass using our periodic table and enter with the proper s.f. If not, enter 0. Do not include units. g/mol In the three blanks, enter the results of the calculation: value (decimal notation with proper s.f.)., units, and substance (in this order).arrow_forwardConverting Concentrations to Different Units Introduction: Depending on how the concentration unit is defined, concentration can serve as a conversion between the amount of a solute and the amount of a solution or solvent. Concentrations can therefore be helpful in a number of stoichiometry issues. The original definition of the concentration unit is frequently preferred because it contains the conversion factor. Today, for chemists, it is suitable to make a solution in the lab using a specific concentration unit like molarity, and then convert the concentration to another unit. Today in the lab, I will prepare a sodium bicarbonate (baking soda) solution with a specific molarity. I will then translate the concentration into molality, mass percent, and mole fraction. Procedure The first step of this lab was to make sure I had everything I needed and that my surroundings were safe. I then looked at an empty beaker weighing paper and a balance. I first took a piece of weighing…arrow_forward
- Aqueous solutions barium chloride and potassium sulfate are combined. 1) Predict the products and write the balanced equation showing the states of each substance. 2) The barium chloride solution has a volume of 100.0 mL and contains 10.00 grams of barium chloride. The molarity of the potassium sulfate solution is 0.574 moles/liter and has a volume of 100.0 mL. Determine the limiting reactant and the mass of each product in grams. 3) Determine how many grams of excess reactant remain. 4) Calculate the molarity of just the potassium ions after the reaction has finished.arrow_forwardHere is a graph of the molarity of thionyl chloride (SOC1₂) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table below. M 0.030- 0.025- 0.0219 0.020- 0.0176 0.015. 0.009703 0.005- 0 1011 seconds Is SOCI, being created or destroyed by the chemical reaction? If SOCI, is being created or destroyed, what is the rate at which it is being created or destroyed 11 seconds after the reaction starts? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. If SOCI, is being created or destroyed, what is the average rate at which it is being created or destroyed. during the first 11 seconds of the reaction? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. 24.513 created O destroyed 30 neither created nor destroyed 0 X 0 ☐ x10 믐 X ロ・ロarrow_forwardA student has a 6.73 liters of a solution that has a molarity of 5.91 M. How many moles of solute are present in this solution? Round your answer to the nearest 0.01 and include units properly abbreviated, but NOT substance!arrow_forward
- What volume, in milliliters, of a 0.185 M solution of KCl contains 2.85 g of the compound? Express the volume to three significant figures and include the appropriate units. HA Value Units volume =arrow_forwardConsider 5.6 mg of sucrose dissolved in water. Identify solute and solvent. Sucrose: Solvent: Which is solvent, which is solute?arrow_forwardFor the following, use proper sig figs, do not include units, and do not place numbers in scientific notation. A simple equation that can be used ONLY for dilutions is M1V1 = M2V2. So if a concentrated solution starts out as 1.36 M and you wish to make 50.0 mL of a 0.286 M solution, how many milliliters of the concentrated solution do you need? How much water will you add?arrow_forward
- The molecular equation for the reaction that take place when two aqueous solutions are mixed is given below. Na2CO3(aq) + MgCl2(aq)→ MgCO:( )+ NaCl( ) a. Predict the physical status of the products, using the solubility rules chart, and indicate below. MgCO3: NaCl: b. Write the balanced molecular equation. c. Write the balanced total ionic equation d. Write the balanced net ionic equationarrow_forward250. mL of water is added to 150. mL of a 2.32 M aqueous solution of glucose. The solution is then heated such that 50.0 mL of water is evaporated. What is the concentration of glucose in the final solution in units of M?arrow_forward1. A 4.24 g sample of a laboratory solution contains 1.42 g of acid. What is the concentration of the solution as a mass percentage? 2. Isopropyl alcohol is mixed with water to produce a solution that is 35.0% alcohol by volume. How many milliliters of each component are present in 645 mL of this solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY