
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:k
esc
1
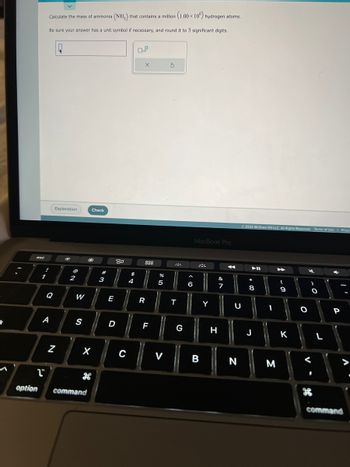
Calculate the mass of ammonia (NH₂) that contains a million (1.00 x 106) hydrogen atoms.
Be sure your answer has a unit symbol if necessary, and round it to 3 significant digits.
T
Q
A
Q
Explanation
N
@
2
W
S
Check
X
option command
#
3
E
80
D
C
$
4
10
X
888
R
F
%
5
V
5
T
G
A
(0)
6
MacBook Pro
B
Y
&
H
ar
7
A
Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privac
U
N
*
+00
8
J
11
I
3
- O
(
9
K
O
V
)
of
O
L
C
P
command
Expert Solution
arrow_forward
Step 1
Answer:
Number of hydrogen atoms given
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Although your textbook lists (he rules for converting an ordinary number to scientific notation, oftentimes students remember such rules better ¡f they put them into their own words. Pretend you are helping your 12-year-old niece with her math homework, and write a paragraph explaining to her how to convert the ordinary number 2421 to scientific notation.arrow_forward1-98 The antifreeze-coolant compound used in cars does not have the same density as water. Would a hydrometer be useful for measuring the amount of antifreeze in the cooling system?arrow_forward1.53 Carry out the following unit conversions. (a) 3.47106g to g , (b) 2.73104L to mL, (c) 725 ns to s, (d) 1.3 m to km.arrow_forward
- In the following scenario, identify which of the statements represents a theory, law, or hypothesis. (a) A student exploring the properties of gases proposes that is she decreases the volume of a sample of gas then the pressure exerted by the sample will increase (b) Many scientists over time have conducted similar experiments and have concluded that pressure and volume are inversely proportional. (c) She proposes that the reason this occurs is that if the volume is decreased, more molecules will collide with a given area of the container walls, causing the pressure to be greater.arrow_forwardWrite the ordinary form of the following numbers: a 2.32 x 10-2, b 9.27 x 104 c 8.96 x 10-4arrow_forward1-89 Consider butter, density 0.860 g/mL, and sand, density 2.28 g/mL. (a) If 1.00 mL of butter is thoroughly mixed with 1.00 mL of sand, what is the density of the mixture? (b) What would be the density of the mixture if 1.00 g of the same butter were mixed with 1.00 g of the same sand?arrow_forward
- Hexane (C6H14, density = 0.766 g/cm3), perfluoro-hexane (C6F14, density = 1.669 g/cm3), and water are immiscible liquids; that is, they do not dissolve in one another. You place 10 mL of each in a graduated cylinder, along with pieces of high-density polyethylene (HDPE, density = 0.97 g/cm3), polyvinyl chloride(PVC, density = 1.36 g/cm3), and Teflon (density = 2.3 g/cm3). None of these common plastics dissolves in these liquids. Describe what you expect to see.arrow_forwardMolecular distances are usually given in nanometers (1 nm = 1 109 m) or in picometers (1 pm = 1 1012 m). However, the angstrom () unit is sometimes used, where 1 = 1 1010 m. (The angstrom unit is not an SI unit.) If the distances between the Pt atom and the N atom in the cancer chemotherapy drug cisplatin is 1.97 , What is this distances in nanometers? In picometers?arrow_forwardIn e-ach of the following pairs, which has the greater mass? (See Table 1.5.) a. 1.0 kg of feathers or 1.0 kg of lead b. 1.0 mL of mercury or 1.0 mL of water c. 19.3 mL of water or 1.00 mL of gold d. 75 mL of copper or 1.0 L of benzenearrow_forward
- 1.89 Imagine that you place a cork measuring 1.30cm4.50cm3.00cm in a pan of water. On top of this cork, you place a small cube of lead measuring 1.15 cm on a side. Describe how you would determine if the combination of the cork and lead cube will still float in the water. Note any information you would need to look up to answer the question.arrow_forwardMagnesium chloride is an important coagulant used in the preparation of tofu from soy milk. Its solubility in water at 20C is 54.6 g/100 g. At 80C, its solubility is 66.1 g/100 g. A mixture is made up of 16.2 g of magnesium chloride and 38.2 g of water at 20C. (a) Is the mixture homogeneous? If it is, how many more grams of magnesium chloride are required to make a saturated solution? If the mixture is not homogeneous, how many grams of magnesium chloride are undissolved? (b) How many more grams of magnesium chloride are needed to make a saturated solution at 80C?arrow_forwardAt 25 C, the density of water is 0.997 g/cm3, whereas the density of ice at 10 C is 0.917 g/cm3. (a) If a soft-drink can (volume = 250. mL) is filled completely with pure water at 25 C and then frozen at - 10 C, what volume does the ice occupy? (b) Can the ice be contained within the can?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning