
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
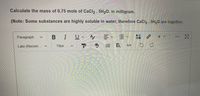
Transcribed Image Text:Calculate the mass of 0.75 mole of CaCI2 . 5H20, in milligram.
(Note: Some substances are highly soluble in water, therefore CaCI2 . 5H20 are together.
Paragraph
BI
of
-" అజి
Ea
-123
Lato (Recom...
19рх ..
</>
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What mass in grams are in 5.32x10^22 molecules of CO2? For some reason, I'm getting confused on how to set up the conversion.arrow_forwardWhich contains more, 6.35 X10E23 molecules of sodium hydroxide or 44.32 g of sodium hydroxide?arrow_forward39.7 mL of a 0.117 M Na2CO3 solution completely react with a 0.150 MHNO3 solution according to the following balanced chemical equation: Na2CO3(aq)+2HNO3(aq)→2NaNO3(aq)+CO2(g)+H2O(l) What mass (in grams) of carbon dioxide is formed? Express your answer with the appropriate units.arrow_forward
- What is the mass of 3.5 x 10^-3 molecules of copper (II) carbonate? Use correct significant figures and units.arrow_forwardA chemist prepares a solution of potassium bromide (KBr) by measuring out 31. mg of KBr into a 100. mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Br anions in the chemist's solution. Be sure your answer is rounded to the correct number of significant digits. alo mol Larrow_forwardA chemist adds 160.0mL of a ×9.310−5/molL magnesium fluoride MgF2 solution to a reaction flask. Calculate the mass in milligrams of magnesium fluoride the chemist has added to the flask. Be sure your answer has the correct number of significant digits. mgarrow_forward
- Given the unbalanced chemical equation, C5H12(l) + O2(g) → CO(g) + H2O(l), which one of the following correctly expresses the mole ratio between CO and oxygen?arrow_forwardIf 31.0 g of MgSO4⋅7H2O is thoroughly heated, what mass of anhydrous magnesium sulfate will remain? Express your answer to three significant figures and include the appropriate units.arrow_forwardHow many grams are in 6.56*; 10 ^ 15 * r molecules of CO?arrow_forward
- Moles of H2C2O4·2H2O? the mass is = 1.32191 grams.arrow_forwardAqueous hydrochloric acid (HCI) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCl) and liquid water (H,O). Suppose 2.19 g of hydrochloric acid is mixed with 3.7 g of sodium hydroxide. Calculate the maximum mass of sodium chloride that could be produced by the chemical reaction. Round your answer to 3 significant digits. Ox10 ?arrow_forwardWhat is the volume (in mL) of isopropanol that contains 7.23 × 1022 molecules of isopropanol? The density of isopropanol is 0.786 g/mL and its molar mass is 60.10 g/mol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY