
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
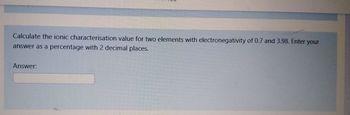
Transcribed Image Text:Calculate the ionic characterisation value for two elements with electronegativity of 0.7 and 3.98. Enter your
answer as a percentage with 2 decimal places.
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- The vacancy concentration for a metal A with an activation energy Q is 2x10-5. Calculate the vacancy concentration for a second metal B with activation energy 1.2Q. (This question has only one correct answer) а. 2.3 x 10-6 b. 4.3 x 10-5 O c. 1.3 x 10-6 d. 3.3 x 10-4arrow_forwardPart A Determine the horizontal component of reaction at the pin A. Neglect the thickness of the members. (Figure 1) Express your answer with the appropriate units. 1 of 1 L. 200 Nm Part B 100 N Determine the vertical component of reaction at the pin A. Express your answer with the appropriate units.arrow_forwardSketch the variation of the vacancy concentration as a function of temperature in iron and mark all salient features on your sketch. The melting temperature of iron is 1539 oC.arrow_forward
- the answer should be equal to 0.842arrow_forwardWhen a cold-worked metal is heat-treated below its melting point over a period of time, which of the following phenomena occur? (This question has more than one correct answer) a. Strength is increased b. Dislocation density decreases c. Grain size increases d. Internal lattice strains decrease e. Ductility is reducedarrow_forwardWhat is cold working process for metals? Why cold working can help improve strength of the metal? Use dislocation theory to explain.arrow_forward
- Sodium chloride (NaCl) has the rock salt crystal structure and a density of 2.17 g/cm³. The atomic weights of sodium and chlorine are 22.99 g/mol and 35.45 g/mol, respectively. (a) Determine the unit cell edge length. nm (b) Determine the unit cell edge length from the radii in the table below assuming that the Nat and Cl- ions just touch each other along the edges. nm Cation Mg2+ Fe2+ Na+ Ionic Radius (nm) 0.072 0.077 0.102 Anion Ionic Radius (nm) CI- 0²- 0.181 0.140arrow_forwardYou are given the density of a metal as 2.07 g/cm³. You are advised the crystallographic structure is fcc, and the atomic mass is 25.5 g/mol. What is the atomic radius of the metal? a. 0.15 nm b. 0.12 nm c. 0.11 nm d. 0.18 nmarrow_forwarddo not provide wrong solutionarrow_forward
- Show that Ni and Cu are totally soluble in one another using Hume-Rothery rules. Atomic radii, electronegativities and crystal structures of Ni and Cu are given below. Ni Cu Crystal Structure FCC FCC Electronegativities 1.9 1.8 r (nm) 0.1246 0.1278arrow_forwardWrite down the Miller-Bravais index of the a-plane of the HCP Zn pure metal and draw the atomic arrangement of the a-plane cross-section with reference to the FCC example below.arrow_forward1. Is there a difference in packing (coordination number) between these two types (FCC and HCP) of structures? 2. Look at the two structures. A small difference in arrangement of atoms causes a dramatic difference in the properties of FCC ductile metals and HCP brittle metals. Can you see it? Try to draw the differences (You can draw the 3-layers separately):arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY