
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
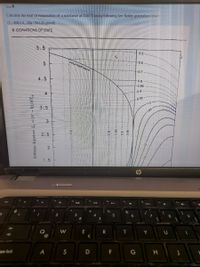
Transcribed Image Text:Soru 4
Calculate the heat of evaporation of a substance at 12.6°Cusing following Lee-Kesler generalized chart
(Te=408.2 K, Mw=58.124 g/mol)
D EQUATIONS OF STATE
5.5
0.5
soturated liquid
-0.6
-0.7
4.5
-0.8
-0.85
-0.9
4
0.95
3.5
05
3
2.5
1.5
IOI
&
6
2
3
#3
7
$
W
E
R
Y
@
€
wbck
S
F
Enthalpy departure Z, = (h" – h)/RT,,
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- In freezing a mole of liquid water at the freezing point, the enthalpy of fusion is 6.01 KiloJoules per mole. Answer the questions that follow. (A). What is the entropy change of this process (in Joules per Kelvin)? Express answer in THREE SIGNIFICANT FIGURES. (B). What is the entropy change of the surroundings for this process (in Joules per Kelvin)? Express answer in THREE SIGNIFICANT FIGURES. (C). What is the total entropy change (or the entropy change of the universe) for this process (in Joules per Kelvin)? Express answer in THREE SIGNIFICANT FIGURES.arrow_forwardONE mole of ethylene expands reversibly from 1L to 20L at a constant temperature of 300 K. Use the van der Waals Equation of State to calculate the following: 1) Work done by the gas 2) the initial pressure of the gasarrow_forwardplease answer the question while referring to the diagram attachedarrow_forward
- The Heat of Solution (AHsol) for CaSO(s) in water is -18.0 kJ/mol. Which of the following is true? More than one of these answer choices are true. O The intermolecular forces between solvent-solute are greater than the sum of the solute-solute and solvent- solvent intermolecular forces. O Crystallization is exothermic. O Dissolution is exothermic.arrow_forwardQ4. Thermodynamic Cycle (Rankin e cycle and Diesel cycle) (a) Steam having dryness fraction of 55% at 70C temperature. Using steam table, calculate: (i) Specific enthalpy h (kJ/kg) Specific entropy s (kJ/kg-K) (ii) (b) Explain saturated liquid line, saturated vapor line and triple pointarrow_forwardNeed asaparrow_forward
- plz help, is wrongarrow_forward(w/ complete process pls) Determine the enthalpy change when 1 mol of SO2 gas is cooled from 538oC to -101oC at atmospheric pressure. The data are:Boiling Point: -5oCMelting Point: -75.5oCLatent Heat of Vaporization: 24,940 J/molLatent heat of Fusion: 7,401 J/molAverage Cp of liquid SO2: 1.28 J/moloCAverage Cp of solid SO2: 0.958 J/moloCCp of gaseous SO2: (kindly provide Cp of gaseous SO2; could be from internet or books)arrow_forwardIf ice homogeneously nucleates at -57.8°C, calculate the critical radius given values of -3.1 x 108 J/m³ and 25 x 10-3 J/m2, respectively, for the latent heat of fusion and the surface free energy 0.9854 x 10-10 m O 0.1284 x 10-5 m O 0.5981 x 10-11 m O 0.7618 x 10-9 marrow_forward
- E Open with Google Docs OCESSES OF PURE SUBSTANCES.pdf EXAMPLES A closed rigid vessel having a volume of 100 m3 contains 10 m3 of saturated liquid water and 90 m3 of saturated water vapor at 1.0 MPaa. Heat is transferred until the vessel filled with saturated vapor. Determine the heat transfer.arrow_forwardDerive the equation in below using the relationships between thermodynamic properties.arrow_forwardThis table displays the vapor pressure of nitrogen at several different temperatures. Use the data to determine the heat of vaporization and normal boiling point of nitrogen.Temperature (K) Pressure (torr)65 130.570 289.575 570.880 102885 1718arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The