
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Calculate the heat absorbed by the dipsidium CaMgSi2O6 when the temperature of the mineral increases from 298 to 1000k. According to the data below:
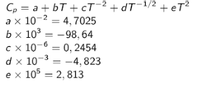
Transcribed Image Text:Cp = a + bT +cT¯2 +dT¯1/2 + eT²
ах 10-2 — 4, 7025
Ьx 103 — -98, 64
сх 10-6 — 0, 2454
d x 10-3
еx 105 — 2, 813
: -4, 823
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 3arrow_forwardEstimate the thermal conductivity of the meat at 35 ° C. (Air content = 62.0% wet basis). Thermal conductivity of the material = AnswerW / m ° Carrow_forwardAir at a dry ball temperature of 75 ° C and 6% RH is passed over the cooling coil so that the air dry ball temperature becomes 30 ° C. How much heat is released from the air in the process? = (kJ / kg).arrow_forward
- D-Q-1 Write T for true Statement or F for False Statement. [1]. Heat transfer: is transit mass between the bodies of materials due to the temperature difference [2]. Conduction: is this phenomenon replay to random motion, translational motion, rotational motion and internal vibrational motion of particles. [3]. The thermal diffusivity represents how fast heat diffuses through a material [4]. A material that has a low thermal conductivity or a low heat capacity will have a large thermal diffusivity. [5]. Heat transfer by radiation does not require the presence of an intervening medium. Convection is the mode of energy transfer between a solid surface and the adjacent liquid or gas that is in motion [6]. [7]. Conduction: is cold transfer from the higher particles activity to lower activity due to the temperature difference [8]. The larger the thermal diffusivity, the non-faster the propagation of heat into the medium [9]. The convection heat transfer coefficient in W/m. [10]. he rate of…arrow_forwardGive a good detailed explanation also along with the correct answer. Not just the answer.arrow_forwardQuestion 7 of 10 Calculate the change in enthalpy of nitrogen when it is heated from 25 to 100 C. Select the correct response: 985.78 J/mol 2185.5 J/mol 3275.4 J/mol 1405.6 J/molarrow_forward
- Gold nanoparticles find applications in photothermal therapy. Their optical properties lead to absorption of light which leads to generation of heat. The optical properties of gold NPs can be tailored by their physical properties - for photothermal therapy, which absorbance wavelength is most preferred; mark all that apply: Light in the UV spectrum Light in the visible spectrum 800-900 nm 600-800 nm Light within the near infrared spectrumarrow_forwardUsing the equipartition theorem, determine the molar specific heat, Cv, of a gas in which each molecule has s degrees of freedom.arrow_forwardThe phases in a distillation column come in contact with each other and exchange __________. a heat b none of these c mass d heat and massarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The