
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Calculate the Entropy of Ammonia in kJ/(kmol K) at T=130 oC and P=1 bar. The reference state is Entropy s=159.3 kJ/(kmol K) at Tref=26.9 oC an Pref=0.01 bar. Assume that in the vapor phase Ammonia behaves like an ideal gas.
Please respond ASAP
Expert Solution
arrow_forward
Step 1
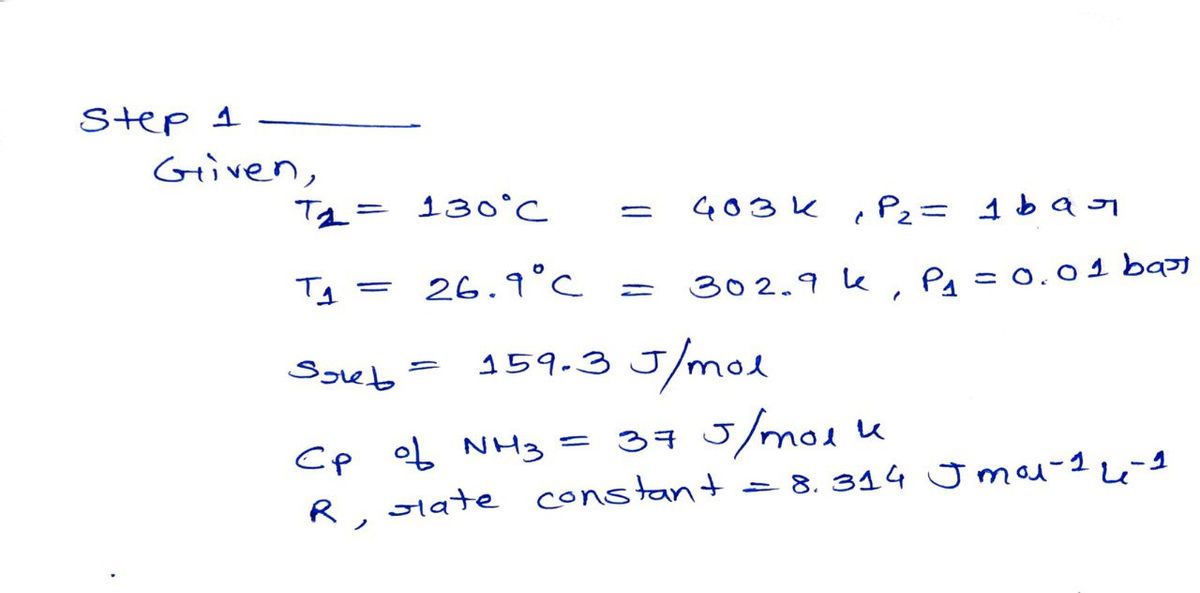
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- t = 165°C, h = 3000 KJ/kgarrow_forwardp = 0.125 MPa, v = 1.3749 m3/kgarrow_forwardthe following data are for steam power plant:Pressure and temperature of steam at boiler exit and turbine inlet (state2)=30 bar and 300°C. Pressure and quality of steam at turbine exit and condenser inlet (state 3) =20 kpa and 95%.Pressure of saturated water at condenser exit and pump inlet (state 4)=20 kpa.Pressure and temperature of water at pump exit and boiler inlet (state1)=30 bar and 100°C.Steam flow rate =15 kg/s.The heat losses from the body of the turbine =100 kW.Determine the thermal efficiency of the steam power plantarrow_forward
- Current Attempt in Progress * Your answer is incorrect. A rigid tank whose volume is 4 m³, initially containing air at 1 bar, 295 K, is connected by a valve to a large vessel holding air at 6 bar, 295 K. The valve is opened only as long as required to fill the tank with air to a pressure of 6 bar and a temperature of 350 K. Assuming the ideal gas model for the air, determine the heat transfer between the tank contents and the surroundings, in kJ. Qcy F i 879.84 eTextbook and Media kJarrow_forward7.arrow_forwardA closed steam room of volume 125 m has a temperature 45 C and has a RH=100%. Calculate: a. The mass of water vapor in the room; b. The person inside decides that it is too hot and lowers the thermostat's temperature to a new temperature of 35 C. What is the mass of water vapor for this temperature? c. How much water vapor will condense, in order to still have RH=100% in the room?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The