
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
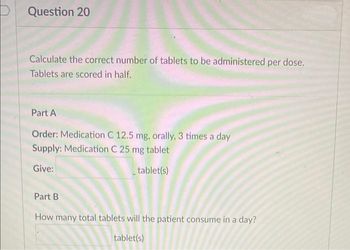
Transcribed Image Text:Question 20
Calculate the correct number of tablets to be administered per dose.
Tablets are scored in half.
Part A
Order: Medication C 12.5 mg, orally, 3 times a day
Supply: Medication C 25 mg tablet
Give:
tablet(s)
Part B
How many total tablets will the patient consume in a day?
tablet(s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You can calculate the cost per dose using the following formula: cost of container /number of doese per container (formula) Calculate the cost per dose for Brand X. Do not include a unit in your answer. Report your answer to three decimal places. Brand X antacid: 2 tablets / 500 mg dose 40 tablets / container Cost: $3.75 / containerarrow_forwardIf you determined the average concentration of vitamin C in the fruit juice to be 0.65 mg/mL, how many milliliters (mL) of juice would you need to drink each day to get the recommended 75 mg of vitamin C? Include the unit and one decimal place in your answer.arrow_forwardA patient is treated with hydroxychloroquine for malaria. The drug is given based on the body mass of the patient. The dosage is 0.274 MG hydroxychloroquine/ 1.65 kg body mass. Calculate the amount (in g) of the drug that would be delivered to a 98.3 kg patient in each dosearrow_forward
- A medical solution had a concentration of 30mg/ml. How many ml would need to be delivered to dispense a total of 150 mg of med?arrow_forwardHow many milliliters of 25.0 mg/L stock solution must be added to a 25.00-mL volumetric flask to produce a 20.0 mg/L solution? Report your answer in decimal form (not scientific notation) with correct sig figs and including one space followed by your units.arrow_forwardA patient needs 25.0 μg of Synthroid each day. The pharmacy only has Synthroid that is 0.025 mg per scored tablet. How many tablets should be taken each day by the patient?arrow_forward
- A student wants to make a 250 mL stock solution with a concentration of 1000 mg Cu/L. The student has an unlimited amount of CuSO4 and DI water. What mass of CuSO4 is needed to make 250 mL the 1000 mg Cu/L solution? Using the stock solution from the previous question, what volume of stock is needed to make a 20 L solution with a concentration of 0.06 mg -Cu/L?arrow_forwardA 110 pound patient is prescribed a maintenance dose of haloperidol. If the dosing regimen is 0.5 mg/kg/day divided into three doses, identify the dose the patient should receive in milligrams (mg).arrow_forwardA sick person requires metronidazole to treat a parasite. Metronidazole should be administered at 7.5 mg/kg body weight. How much metronidazole would a 170 lb patient need (1 kg = 2.2 lb)?arrow_forward
- Assume the recommended single dose of ibuprofen for children over the age of two is 4.5 mg per pound (mg/lb) 4.5 mg per pound (mg/lb) of body weight. Use this guideline to calculate a single dose in milligrams for a five‑year‑old child who weighs 51Lb.arrow_forwardA patient is prescribed 100mg/day of antibiotic for two weeks. The antibiotic is available in vials that contain 20mg/vial of the drug. How many vials are necessary for the entire treatment?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY