
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
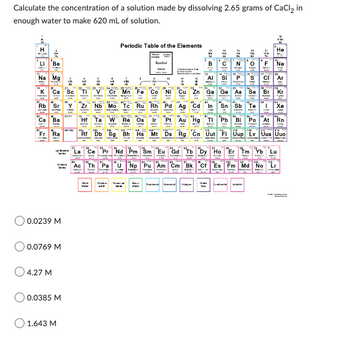
Transcribed Image Text:Calculate the concentration of a solution made by dissolving 2.65 grams of CaCl₂ in
enough water to make 620 mL of solution.
LI 'Ве
Na Mg
Rb Sr
Cs Ba
Fr Ra
0.0239 M
0.0769 M
4.27 M
0.0385 M
1.643 M
Sc
3311
Periodic Table of the Elements
M
_294 29, 1245 24 24 25 26 25 27
Cr Mn
42
Zr Nb Mo Tc
Symbol
B
}
Al
C
si
N
Fe Co NI Cu Zn Ga Ge A
F
nsto!_o}! |} }&{}}}\
CI
Ru Rh Pd Ag Cd In
Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
Rf Db Sg Bh Hs Mt Ds Rg Cn Uut FI Uup Lv Uus Uuo
Co "Pr_ "Nd
"Pr Nd Pm Sm
Gd
Dy Ho
"Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No
"He
F Ne
Se Br Kr
Tm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemist starts with 25.0 mL of a 0.20 M NaCl solution and dilutes it to 500 mL. What is the concentration of NaCl in the new solution? 0.060 M 0.080 M 0.020 M 0.040 M What is the molarity of a KCl solution made by diluting 75.0 mL of a 0.200 M solution to a final volume of 100. mL? 0.267 M 6.67 M 0.150 M 0.200 M Consider the following sample data or measurement. 167, 180, 188, 177, 181, 185, 189 Is 167 an outlier at 90% confidence interval?arrow_forwardA student dissolved 5.00 g of Co(NO3)2 in enough water to make 100. mL of stock solution. He took 4.00 mL of the stock solution and then diluted it with water to give 275. mL of a final solution. How many grams of NO3- ion are there in the final solution? A) 0.0 247 g B) 0.0 493 g C) 0.0 678 g D) 0. 136 g E. 0.475 g 4arrow_forwardUse the References to access important values if needed for this question. A student weighs out a 6.27 g sample of AlBr3, transfers it to a 100. mL volumetric flask, adds enough water to dissolve it and then adds water to the 100. mL tick mark. What is the molarity of aluminum bromide in the resulting solution? Molarity M %3Darrow_forward
- A student prepares a solution by dissolving 9.23 g of solid KOH in enough water to make 300.0 mL of solution. Calculate the molarity of K+ ions in this solution.arrow_forwardA chemist prepares a solution of iron(II) bromide (FeBr,) by measuring out 97.1 mg of FeBr, into a 100. mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Br anions in the chemist's solution. Be sure your answer is rounded to 3 significant digits. olo mol x10 Ararrow_forward9) A solution is prepared by dissolving 0.148 g of potassium phosphate (K3PO4) in water. The final volume of the solution is 200.0 mL. The concentration of K+ ion in the solution is a) 6.97 x 10-4 M d) 1.05 х 10-2 М b) 1.16 x 103 м e) 0.740 M с) 3.49 х 10-3 мarrow_forward
- A 20.91 mL solution containing 1.678 g Mg(NO3), is mixed with a 26.79 mL solution containing 1.215 g NaOH. Calculate the concentrations of the ions remaining in solution after the reaction is complete. Assume volumes are additive. If a species fully precipitates, type 0. Be sure your answer has the correct number of significant digits. M Mg 2+ ΜΝΟ, M Na M OH x10 X Sarrow_forwardA student dissolved 6.00 g of Co(NO3)2 in enough water to make 100. mL of stock solution. He took 4.00 mL of the stock solution and then diluted it with water to give 275. mL of a final solution. How many grams of NO3- ion are in the final solution? 0.0296 g 0.0592 g 0.0814 g 0.163 garrow_forwardA solution of Ca(NO3)2 at 1.903 M is diluted from its original volume of 0.7865 L by adding 0.6115 L. What is the resulting concentration of this solutionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY