
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Transcribed Image Text:15 Question
e See page 810
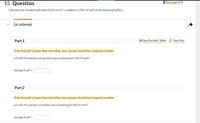
Calculate the change in pH when 0.254 mol H* is added to 1.00 L of each of the following buffers.
1st attempt
Part 1
l See Periodic Table O See Hint
If the final pH is lower than the initial, your answer should be a negative number.
a 0.560 M solution of pyridine (py) containing 0.500 M pyH*
change in pH =
Part 2
If the final pH is lower than the initial, your answer should be a negative number.
a 0.560 M solution of aniline (an) containing 0.920 M anH*
change in pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the pH during the titration of 10.00 mL of 0.400 M hypochlorous acid with 0.500 M NaOH. First what is the initial pH (before any NaOH is added)? The Ka for HOCl is 3.0 x 10-8 M.(THE ANSWER IS 4.96) I DONT NEED THIS ABOVE QUESTIONS DONE!!!! THE QUESTIONS BELOW RELATE TO IT THOUGH!!!! What is the pH after 4.80 mL of NaOH are added? What is the pH at the equivalence point? What is the pH after 9.80 mL of NaOH are added?arrow_forwardSet up an ice table, find the Kb expression and find the initial concentration for C6H5NH3+arrow_forwardPlease don't provide handwriting solutionarrow_forward
- Determine the resulting pH when 0.003 mol of solid NaOH is added to a 100.0 mL buffer containing 0.13 M HClO and 0.37 M NaClO. The value of Ka for HClO is 2.9 × 10⁻⁸. Determine the moles of the ractant and product after the reaction of the acid and base. (Similar to ICE format but is instead Before (mol), Change (mol), and After (mol) Determine the ICE table for HClO (aq) + H2O - H3O+ + ClO- (aq) Fill in Ka= ? = 2.9 * 10-8 Calculate pHarrow_forwardPlease get it right please ??arrow_forwardplease help with both partsarrow_forward
- Which of the following, if dissolved in 1.0 L of pure water, will produce a buffer solution? 0.1 mole HCL + 0.1 mole KCl 0.1 mole NaH2PO4 + 0.1 mole Na2HPO4 0.1 mole NaCl + 0.1 mole KCl 0.1 mole of H3O+ + 0.1 mole OH- I am guessing option 2, but need clarification please!arrow_forwardPparrow_forwardA solution contains 0.312 M HA (K₂ = 2.20-10-7) and 0.680 M NaA. What is the pH of this solution? What is the pH of this solution after 0.212 mol of HCI are added to 1.00 L of this solution? What is the pH of this solution after 0.424 mol of HCI are added to 1.00 L of this solution? Question Help: Video Submit Question [11arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY