
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
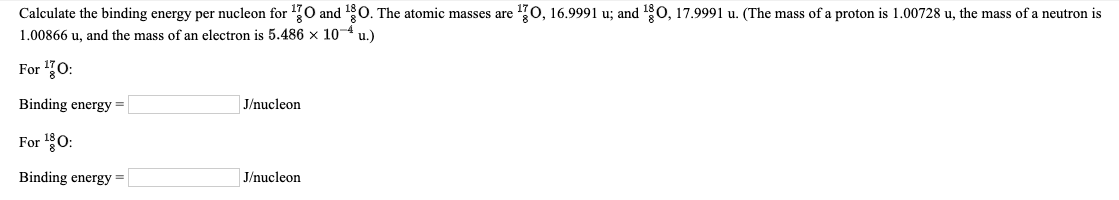
Transcribed Image Text:Calculate the binding energy per nucleon for 170 and 10. The atomic masses are 70, 16.9991 u; and 180, 17.9991 u. (The mass of a proton is 1.00728 u, the mass of a neutron is
1.00866 u, and the mass of an electron is 5.486 x 104 u.)
For 0:
Binding energy =
J/nucleon
For 0:
Binding energy
J/nucleon
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Similar questions
- Calculate the following for 64Zn (actual mass = 63.9291 amu): (a) binding energy in kJ/mol; (b) binding energy in MeV/nucleon. Consider the mass of e for this process. The respective atomic masses are 0.00054858 amu for e-, 1.0073 amu for p+, and 1.0087 amu for no.arrow_forwardCalculate the binding energy per nucleon of in kJ/mol. The required masses (in g/mol) are: 15.00011 g/mol 1.00783 g/mol 1.00867 g/mol kJ/mol nucleonsarrow_forwardWhat is the binding energy per nucleon for Kr-93, which has a mass of 92.93113 amu. please show work:) It helps me better understand the process of working irbourarrow_forward
- What is the binding energy in kJ/mol Cl for chlorine-35? The required masses (g/mol) are: H= 1.00783;n= 1.00867:35C1= 34.96885 kJ/molarrow_forwardI need help balancing these nuclear equations. Thank youarrow_forwardFind the activity of a sample containing 5.69 10-6 g of 60-27 Co (T1/2 = 5.27 years).arrow_forward
- The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the binding 60 energy (in J) of a Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu. Speed of light = 27 3.00 × 108 m/s.) O 2.74 x 10-16 -28 9.12 x 107 O 2.74 x 10-19 -13 4.94 x 107 8.20 × 10-11arrow_forward2 Calculate the binding energy (J/nucleon) for Uranium-235 (molar mass= Mass of proton = 1.67262192369E-27 kg Mass of a neutron 1.67492749804E-27 kg Mass of an electron = 9.1093837015E-31 kg Speed of light = 299792458 m/s NA = 6.022E23 particles / mol = Enter a number - no units. 235.04393 g/mol).arrow_forward3. PuO2 has a density of 11.5 g cm- and a specific heat capacity of 330 J kg' K-1. A particular old sample (i.e. one that has 'partially decayed*) of PuO2 produces 0.440 kW kg' of heat due to internal radioactive decay. > The power output of a 2.00 cm' sample of this partially decayed' PuO2 is therefore 10.2 J/s. PuO, melts at 2673 K and its latent heat of fusion is 245 kJ kg:!. If we place our 2.00 cm block in a well insulated and heat resistant container when it is at 300 K, then how long is it before the whole block is melted?arrow_forward
- 18. Samarium-151 is a beta emitter enba ue aUM the product nucleus. 19. How much of a 24-gram sample of Radium-226 will remain unchanged at the end of three half-life periods? Accessibility: Good to go a iparrow_forwardWhat is the binding energy in kJ/mol Cu for copper-65? kJ/mol 29 H+ 36;n- Cu 65 The required masses (g/mol) are:H= 1.00783 ;on= 1.00867 ;2 Cu = 64.92780arrow_forwardWhat is the nuclear binding energy per nucleon, in joules, for Mg-25 (atomic mass 24.985839 amu). [Data: H-1 (atomic mass) = 1.007825 amu; neutron (mass) = 1.008665 amu; 1 kg = 6.022 x 10e26 amu; c = 3.00 x 10e8 m/s]arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY