
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
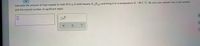
Transcribed Image Text:Calculate the amount of heat needed to melt 45.0 g of solid hexane (C,H,4) and bring it to a temperature of - 49.2 °C. Be sure your answer has a unit symbol
and the correct number of significant digits.
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Try Again Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. Calculate the amount of heat needed to boil 127. g of benzene (CH), beginning from a temperature of 59.1 °C. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol. 4.53 kJ x10 X Śarrow_forward(CONTINUED ON NEXT PAGE) 4. Let's say that the full volume of your flask is 325 ml. You heat the flask of air in a hot water bath at 89.0°C according to your thermometer. You then inverti into the cold water bath at 5.0°C Water enters the flask as the volume of the gas shrinks. a) Theoretically, what should the volume of the gas be after it sits in the cold water bath? b) You find that during your experiment that 65.0 mL of water has entered the flask. What is the experimental volume of the gas? c) What is your percent error for this measurement?arrow_forwardplease answer quicklyarrow_forward
- While heating two different samples of water at sea level one boils at 102°C and one boils at 99.2°C calculate the percentage error for each sample from the theoretical hundred degrees Celsiusarrow_forwardIn Ray Bradbury’s 1953 novelFahrenheit 451, 451°F is said to be the temperature at which books,which have been banned in the story, ignite. Convert 451°F to theCelsius scale.arrow_forwardCalculate the amount of heat needed to melt 140. g of ice (H₂O) and bring it to a temperature of 23.2 °C. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol. □ x10 Xarrow_forward
- Please don't provide handwritten solutionarrow_forwardA cryogenic vacuum pump works by condensing vapors onto some absorbent medium. This is an efficient and clean way to pump a system in a research environment. The term cryo means cold, which indicates that these types of vacuum pumps contain a refrigerant cycle to cool the internal parts. The temperature difference between the inside and outside of a typical cryogenic pump is AT = 312°C. Derive an expression to convert this difference into Fahrenheit and express the answer.arrow_forwardConvert 125 °C to Kelvinarrow_forward
- Calculate the amount of heat needed to melt 16.0 g of solid octane (C8H₁8) and bring it to a temperature of 58.3 °C. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol. x10 Xarrow_forwardCalculate the joules released when 165 g of stream condenses at 100 celsius and the liquid cools to 25 celsius. Express your answer to three significant figures and include the appropriate units.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY