
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
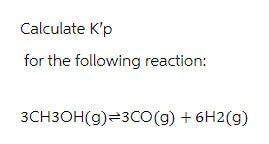
Transcribed Image Text:Calculate K'p
for the following reaction:
3CH3OH(g) 3CO(g) + 6H2(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 4arrow_forwardInterconvert K and K values. Calculate Ko for the following reactions at the indicated temperature. (a) 2 NOCI(g) 2 NO(g) + Cl₂(g) K = 2.69x10-³ at 560 K Kp = (b) NHAl(s)NH₂(g) + HI(g) Kc = 5.59×10-4 at 634 K Kp =arrow_forward8.2 mol NH3 are placed in a 10.5 L container at some temperature and allowed to come to equilibrium as per the reaction below. At equilibrium, 2.2 moles remained. What is the value of Kc for the following reaction at the temperature of the experiment? 2 NH3(g) N2(g) + 3 H2(g)arrow_forward
- Enter your answer in the provided box. Consider the following reaction at a high temperature. Br2(g) 2Br(g) When 1.30 moles of Br2 are put in a 0.710-L flask, 1.90 percent of the Br2 undergoes dissociation. Calculate the equilibrium constant Ke for the reaction.arrow_forwardAt 475 oC, Keq = 0.230 for the reaction: 1/3 NO2(g) + 1/3 N2O(g) (equilibrium arrows) NO(g) (a) What is the value of Keq for the reaction NO(g) 1/3 NO2(g) + 1/3 N2O(g)?Keq = .(b) What is the value of Keq for the reaction NO2(g) + N2O(g) 3 NO(g)?Keq = .(c) What is the value of Keq for the reaction 3 NO(g) NO2(g) + N2O(g)?Keq = .arrow_forwardCalculate the mass of PCl5, that must be heated in a 1.0L flask at 250 oC in order to produce enough chlorine to give an equilibrium concentration,[Cl2]eq. of 0.20 mol/L? PCl5(g) <——> PCl3(g) + Cl2(g) Kc = 0.480arrow_forward
- Consider the following equilibrium for which H = -114.44: 4 HCl(g) + O2(g) 2 Cl2(g) + 2 H2O(g) How will each of the following changes affect an equilibrium mixture of the 4 gases in this reaction? (a) A catalyst is added to the reaction mixture. which will happen? the equilibrium will not shift the equilibrium will shift towards product but keq will not change the equilibrium will shift towards reactant but keq will not change the equilibrium will shift towards product and keq will increase the equilibrium will shift towards reactant and keq will decrease (b) The total pressure of the system is increased by adding a noble gas. (c) Cl2(g) is removed from the system.arrow_forwardAcetaldehyde (CH3CHO) is an important chemical both industrially and biologically. For instance, it is a (somewhat toxic) intermediate in the body's metabolism of ethanol into acetic acid, and thus is possibly implicated in the "hungover" symptoms of someone who has had too much to drink the night before. In aqueous solution, it establishes an equilibrium with a hydrated form, shown below. CH3CHO (aq) + H2O (l) <--> CH3CH(OH)2 (aq) You start with an aqueous sample, already at equilibrium, with the CH3CH(OH)2 (the hydrated form) at a concentration of 2.60 M. You have no information about how much, if any, of the anhydrous form (CH3CHO) is initially in the flask. If you add 2.0 M of CH3CHO to the reaction flask, and as the equilibrium is being restored the amount of CH3CH(OH)2 changes by 1.13 M, what is the final amount of CH3CHO?arrow_forwardIn the reaction: 2A ⇆ B + 3C For the reaction, ΔH = -23 kcal/mol and Keq = 26 X 10 -26 Is the reaction exothermic or endothermic? Are products or reactants favored? Using Le Chatelier’s Principle, what happens to the equilibrium if the concentration of C is increased? Using Le Chatelier’s Principle, what happens to the equilibrium if the concentration of A is increased?arrow_forward
- What is the equilibrium constant expression for the reaction:3 Fe(s) + 4 H2O (g)⟷ Fe3O4 (s) + 4 H2 (g)arrow_forwardAfter equilibrium is reached in the reaction of 6.30 g H2 with 150. g I2, analysis shows that the 1.00 L flask contains 64.0 g of HI. How many moles of H2, I2, and HI are present in this equilibrium mixture? What is the Keq for this reaction?arrow_forwardConsider the following equilibrium for which H = -114.44: 4 HCl(g) + O2(g) 2 Cl2(g) + 2 H2O(g) How will each of the following changes affect an equilibrium mixture of the 4 gases in this reaction?(a) O2(g)) is added to the system. which will happen? the equilibrium will not shift the equilibrium will shift toward product but Keq will not change the equilibrium will shift toward reactant but Keq will not change the equilibrium will shift toward product and Keq will increase the equilibrium will shift toward reactant and Keq will decrease (b) The reaction mixture is cooled. the equilibrium will not shift the equilibrium will shift toward product but Keq will not change the equilibrium will shift toward reactant but Keq will not change the equilibrium will shift toward product and Keq will increase the equilibrium will shift toward reactant and Keq will decrease (c) The volume of the reaction vessel is reduced by 50%. (d) A catalyst is added to the reaction mixture. (e)…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY