
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
I am having trouble finding the Kp values here. The free energy of the reaction should be = 2*51.3-2*86.6=-70.6 kJ/mol
exponent for Kp should be:
(-70.6 kJ/mol * 1000 J/kJ)/(-8.134 J/(mol*K) *298.15K) = 29.1116
so Kp=e29.1116=4.395x1012 but this evidently not correct. Please help?
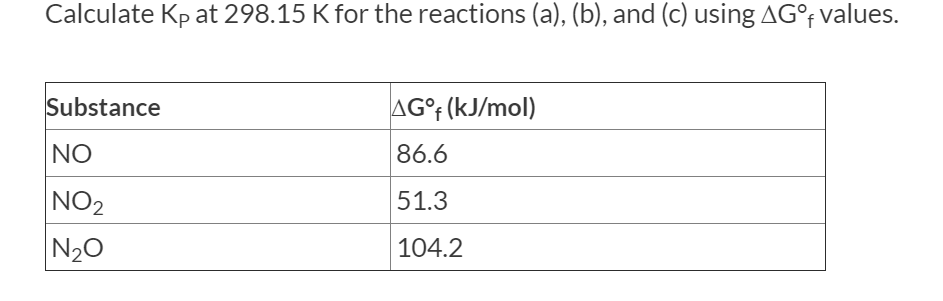
Transcribed Image Text:Calculate Kp at 298.15 Kfor the reactions (a), (b), and (c) using AG°f values.
Substance
AG°F (kJ/mol)
NO
86.6
NO2
N20
51.3
104.2
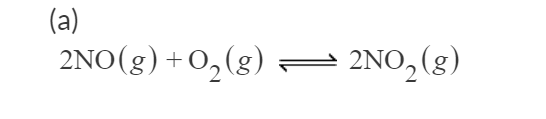
Transcribed Image Text:(a)
2NO(g) +0,(g) 2NO, (g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Is the following reaction spontaneous as written? Explain. Do whatever calculation is needed to answer the question. SO2(g)+H2(g)H2S(g)+O2(g)arrow_forwardElemental boron, in the form of thin fibers, can be made by reducing a boron halide with H2. BCl3(g) + 3/2 H2(g) B(s) + 3HCl(g) Calculate H, S, and G at 25 C for this reaction. Is the reaction predicted to be product favored at equilibrium at 25 C? If so, is it enthalpy driven or entropy driven?arrow_forwardThe equilibrium constant for a reaction decreases as temperature increases. Explain how this observation is used to determine the sign of either H or S.arrow_forward
- Consider the reaction NH4+(aq) H+(aq)+NH3(aq) Use G f for NH3(aq) at 25C=26.7 kJ/mol and the appropriate tables to calculate (a) G at 25C (b) Ka at 25Carrow_forwardConsider the decomposition of red mercury(II) oxide under standard state conditions.. 2HgO(s,red)2Hg(l)+O2(g) (a) Is the decomposition spontaneous under standard state conditions? (b) Above what temperature does the reaction become spontaneous?arrow_forwardCalculate G at 355 K for each of the reactions in Question 17. State whether the reactions are spontaneous.arrow_forward
- Calculate K at 25°C for each of the reactions referred to in Question 32. Assume smallest whole-number coefficients.arrow_forwardFor the system 2SO3(g)2SO2(g)+O2(g) K=1.32 at 627. What is the equilibrium constant at 555C?arrow_forwardConsider the reaction CO(g)+H2O(g)CO2(g)+H2(g) Use the appropriate tables to calculate (a) G at 552C (b) K at 552Carrow_forward
- For the reaction 2Cu(s)+S(s)Cu2S(s) H and G are negative and S is positive. a At equilibrium, will reactants or products predominate? Why? b Why must the reaction system be heated in order to produce copper(I) sulfide?arrow_forwardThe free energy change, G, for a process at constant temperature and pressure is related to Suniv and reflects the spontaneity of the process. How is G related to Suniv? When is a process spontaneous? Nonspontaneous? At equilibrium? G is a composite term composed of H, T, and S. What is the G equation? Give the four possible sign combinations for H and S. What temperatures are required for each sign combination to yield a spontaneous process? If G is positive, what does it say about the reverse process? How does the G = H TS equation reduce when at the melting-point temperature of a solid-to-liquid phase change or at the boiling-point temperature of a liquid-to-gas phase change? What is the sign of G for the solid-to-liquid phase change at temperatures above the freezing point? What is the sign of G for the liquid-to-gas phase change at temperatures below the boiling point?arrow_forwardGiven the following data at a certain temperature, 2N2(g)+O2(g)2N2O(g)K=1.2 10 35 N2O4(g)2NO2(g)K=4.6 10 3 12 N2(g)+O2(g)NO2(g)K=4.1 10 9 calculate K for the reaction between one mole of dinitrogen oxide gas and oxygen gas to give dinitrogen tetroxide gas.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning