
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
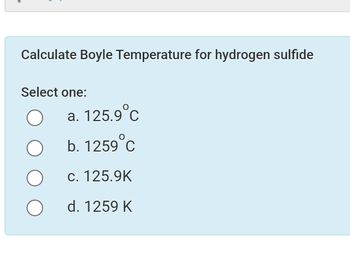
Transcribed Image Text:Calculate Boyle Temperature for hydrogen sulfide
Select one:
a. 125.9°C
b. 1259°C
C. 125.9K
d. 1259 K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- when hydrazine reacts with oxygen. nitrogen gas and steam are formed how much heat is evolved or absorbed if 1.683 L of steam at 125OC and 772mm Hg are obtainedarrow_forward24.34 Draw the product of each of the following reactions. piease show Allarrtw pasheng wedranonsarrow_forwardO ||| Use the observation in the first column to answer the question in the second column. soft C W W Microsoft Microsoft 6.52.210... OSTATES OF MATTER Understanding the connection between vapor pressure, boiling... esc The enthalpy of vaporization of Substance E is smaller than that of Substance F. observation At 1 atm pressure, Substance A boils at -47. °C and Substance B boils at -8. °C. At 72 °C, Substance C has a vapor pressure of 80. torr and Substance D has a vapor pressure of 100. torr. Explanation ! 1 Q 2 Check 9,088 W question At any temperature where both substances are liquid, which has the higher vapor pressure? 280 Substance E Substance F Neither, E and F have the same vapor pressure. It's impossible to know without more information. Which has a higher enthalpy of vaporization? Substance A Substance B Neither, A and B have the same enthalpy of vaporization. It's impossible to know without more information. Which has a higher enthalpy of vaporization? #m 3 Substance C…arrow_forward
- 1a. Calculate the pressure of a sample of 1 mole of argon gas in a 0.1500 L flask at -10.2 ℃ using the ideal gas law and the Van der Waals equation. The a value for argon is 1.34 (L 2 ∙ atm/mol2 ) and the b value is 0.0322 (L/mol). Show all work Ideal pressure= ? real pressure =? 1b.Why is there a difference in the real gas pressure and the ideal pressure? Explain what assumption(s) of the kinetic molecular theory of gases break down under these conditions.arrow_forward21) write the Nobel gas configuration for bromine. You do not need to use subscriptsarrow_forward1. Calculate AHf NI2 Oz given 2Ni Is) t 5 Ozlq) 7 Ni 2 O3 LS) mass of ca lorime tek SpeciPic heat of calorimeter - mass af water specific heat o p watek - 4.18 5/9.c #oF grams Ni used t Of grams oe Oz initnal temperatue Final temperatuze 6000 1200 g 19.33g 7.999 21.45 °C 24.43°Carrow_forward
- Theoretically, when an ideal gas in a closed container cools, the pressure will drop steadilyuntil the particles essentially stop moving. At what temperature should this occur? a: -273 K b: 0 K c: -460 ºC d: 0ºCarrow_forwardHow many grams of Hg can be vaporized using 29330J of energy?arrow_forwardC Chegg Search x C A Haber Process Reactor Cont x G AL 35.0 "C, a 12.0L vessel is f x pp.101edu.co mail O YouTube O Maps a AMAZON A Translate onights 8 usCis b BATERBLY C CHEGO > KATAPULK CUBA SUPERMARKET23 + Essay Writing Ser. G calculator - Googl. tO Reading List Question 22 of 25 Submit At 35.0 °C, a 12.0 L vessel is filled with 6.25 moles of Gas A and 6.00 moles of Gas B. What is the total pressure? atm 2 3 4 C 7 9 +/- x 100 MacBook Pro 3 6 2 4 7 8 R. T Y F G H J K L Marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning