
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
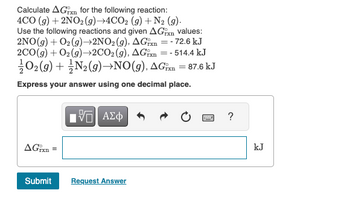
Transcribed Image Text:Calculate AGxn for the following reaction:
4CO (g) + 2NO₂(g) →4CO2 (g) + N₂ (9).
=-
Use the following reactions and given AGrxn values:
2NO(g) + O₂(g) →2NO₂ (g), AGxn - 72.6 kJ
2CO(g) + O₂(g) →2CO2 (g), AGxn=-514.4 kJ
O2(g) + N₂(g) →NO(g), AGixn = 87.6 kJ
Express your answer using one decimal place.
AGxn
Submit
VE ΑΣΦ
Request Answer
www
Henry
?
kJ

Transcribed Image Text:When ethanol (CH3 CH₂OH) is combusted, such as when in a gasoline blend, the following reaction occurs:
Based on the standard free energies of formation given in the table below, what is the standard free energy change for this reaction?
Express your answer to one decimal place and include the appropriate units.
► View Available Hint(s)
AG° =
Submit
LO
■
μA
1370.4
4
Previous Answers
kJ
wwwww
?
2CH3 CH₂OH(1) + 602(g) →4CO2(g) + 6H₂O(g)
X Incorrect; Try Again; 5 attempts remaining
Substance
CH3CH₂OH(1)
O₂ (g)
CO₂(g)
H₂O(g)
AG
(kJ/mol)
-174.9
0
-394.4
-228.6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When the substances in the equation below are at equilibrium at pressure Pand temperature T, how can the equilibrium be shifted to favor the products?CuO(s) + H2(g) Cu(s) + H2O(g)Change in enthalpy = –2.0 kJ.arrow_forwardWhat is AG° (in kJ/mol) for the autoionization of water at 25°C? R = 8.314462 J/K-mol IMPORTANT: When entering your answer: • Enter the number only (no units) • Do not leave any spaces • Include the sign if your answer is negative (not necessary for positive values) • Use a leading zero before the decimal when necessary Report your number to 1 decimal place (regardless of the significant figures) • Correctly round your answer to the 1st decimal place (margin of allowed error is only ± 0.1, so always use un-rounded numbers in your calculations). If the last digits are zeros they will not display once you click away, but the answer will still be marked correct. Examples: 12.3 or -0.4arrow_forwardPart b For the reaction given in Part A, how much heat is absorbed when 2.60 mol of A reacts? And part c said At what temperature Teq do the forward and reverse corrosion reactions occur in equilibrium?arrow_forward
- Fine particles of metallic iron can be injected underground to remediate pollution of underground aquifers by the industrial solvent trichloroethane. In one experiment, 2400L of an aqueous emulsion containing ~480 kg of Fe(0) consumed 17.0 kg of trichloroethane in 5 months. Write a balanced reaction using H2O and H+ to complete the balancing. What percentage of injected iron was used by this reaction in 5 months? Fe + C2HCl3 ---> Fe2+ + C2H4 + Cl-arrow_forwardCalculate ArH for the following reaction: CaO(s)+CO2(g)→CaCO3(s) Use the following reactions and given >ArH"s Ca(s)+CO2(g)+1/202(g) →CaCO3(s), ArH= -812.8 kJmol-1 2Ca(s)+02(g) →2CaO(s), ArH= -1269.8 kJmol-1 ArH=-177.9 kJ mol-1 ArH= 177.9 kJmol-1 ArH=327.9 kJmol-1 ArH=-327.9 kJmol-1arrow_forwardGiven the thermodynamic properties below, determine the standard enthalpy change, ∆H˚, for the dissolution of calcium hydroxide. Give your answer to one decimal place in kJ/mol. 1 Ca(OH)2 (s) ⇌ Ca2+ (aq) + 2 OH– (aq)arrow_forward
- The following reaction has a AG° value of +8.43 kJ/mol at 25 °C. HA(aq) + H20(1) =H;O*(aq) + A (aq) Calculate the KA for the acid HA.arrow_forwardFor each reaction, write the chemical formulae of the oxidized reactants in the space provided. Write the chemical formulae of the reduced reactants in the space provided. reactants Mg oxidized: CuCl, (ag) + Mg(s) - MgCl, (ag) + Cu(s) reactants CuCl, reduced: reactants oxidized: 2AGNO, (ag) + Mg(s) 2Ag(s) + Mg(NO,), (aq) → reactants reduced: reactants oxidized: Feso, (ag) + Mg(s) → Fe(s) + MgSO, (aq) reactants reduced:arrow_forwardDetermine the standard Gibbs free energy of reaction, in kJ/mole, for the reaction of aqueous sodium carbonate with hydrochloric acid. G values for the reactants and products are given below. NaCO(aq) + 2 HCl(aq) 2 NaCl(aq) + HO() + CO(g) NaCO(aq) -1051.6HCl(aq) -131.2NaCl(aq) -393.1HO() -237.1CO(g) -394.4arrow_forward
- Give correct detailed Solutionarrow_forwardConsider the unbalanced equation for the neutralization of acetic acid: αHC2H3O2(aq)+βBa(OH)2(aq) →γH2O(l)+δBa(C2H3O2)2(aq) Balance the equation. Give your answer as an ordered set of numbers α, β, γ, δ. Your answers should reflect the lowest whole-number ratio of coefficients.arrow_forwardHow many Calories should there be in three grams of pure sucrose?A.4B. 12C. 18D. 8E. None of the above.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY