
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Q.61
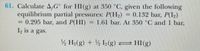
Transcribed Image Text:61. Calculate A,G° for HI(g) at 350 °C, given the following
equilibrium partial pressures: P(H.)
= 0.295 bar, and P(HI)= 1.61 bar. At 350 C and 1 bar,
I, is a gas.
= 0.132 bar, P(I,)
½ H»(g) + ½ I½(g) = HI(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Tennis balls N2(g) , each ball : V= 144cm3; 0.33g of N2(g), P inside each ball ?arrow_forwardDetermine the molar mass (also known as molecular weight or molecular mass) of the following molecule: Mg1S2(C1O2)2 Enter your answer as a number with at least 3 decimal places.arrow_forwardNeed help with homeworkarrow_forward
- A Chemistry 20 student uses a thermometer and a hot plate and measures the boiling point of ethyl alcohol to be 74.3 ºC. Then, she looks in a reference book and finds that the actual boiling point of ethyl alcohol is 78.4 ºC. What is her percent error?arrow_forwardExample 2 The decimal 251.737 is read "two hundred fifty-one and seven hundred thirty-seven thousandths." ( a. Write in words the weight of potassium in a 150-pound body. (3 b. Write in words the weight of the amount of copper in a 150-pound body. c. Which element would have a weight of six hundred-thousandths of a pound? a. Write the number 9,467.00624 in words. b. Write the number 35,454,666.007 in words. c. Write the following number using decimal notation: four million sixty-four and seventy-two ten-thousandths. d. Write the following number using decimal notation: seven and forty-three thousand fifty-two millionths. Converting Decimals to Fractions Note that each of the decimals you have encountered thus far in this activity end after a few digits. Decimals such as these are referred to as terminating decimals. When a terminating decimal is written as a fraction or mixed number, the denominator corresponds to the place value of the rightmost digit in the decimal. This leads to the…arrow_forwardWhat is -30°F in K? O 30K ЗОК O 303 K О-30 К 0239 К 04Karrow_forward
- 20 cm3 of a gaseous hydrocarbon required 90 cm3 of oxygen for complete combustion. Both volumes were measured under the same conditions.The hydrocarbon must be?arrow_forwardIf X and Y are quantities that are related to each other by inverse proportion, what will the value of X become when the value of Y is increased by a factor of five? Select one: a. It will also increase by a factor of 5. b. It will decrease by a factor of 5. c. It will stay the same. d. There is no way to tell.arrow_forwardMatter that has a uniform and unchanging composition is know as a... a. compound b.mixture c. substancearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY