
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
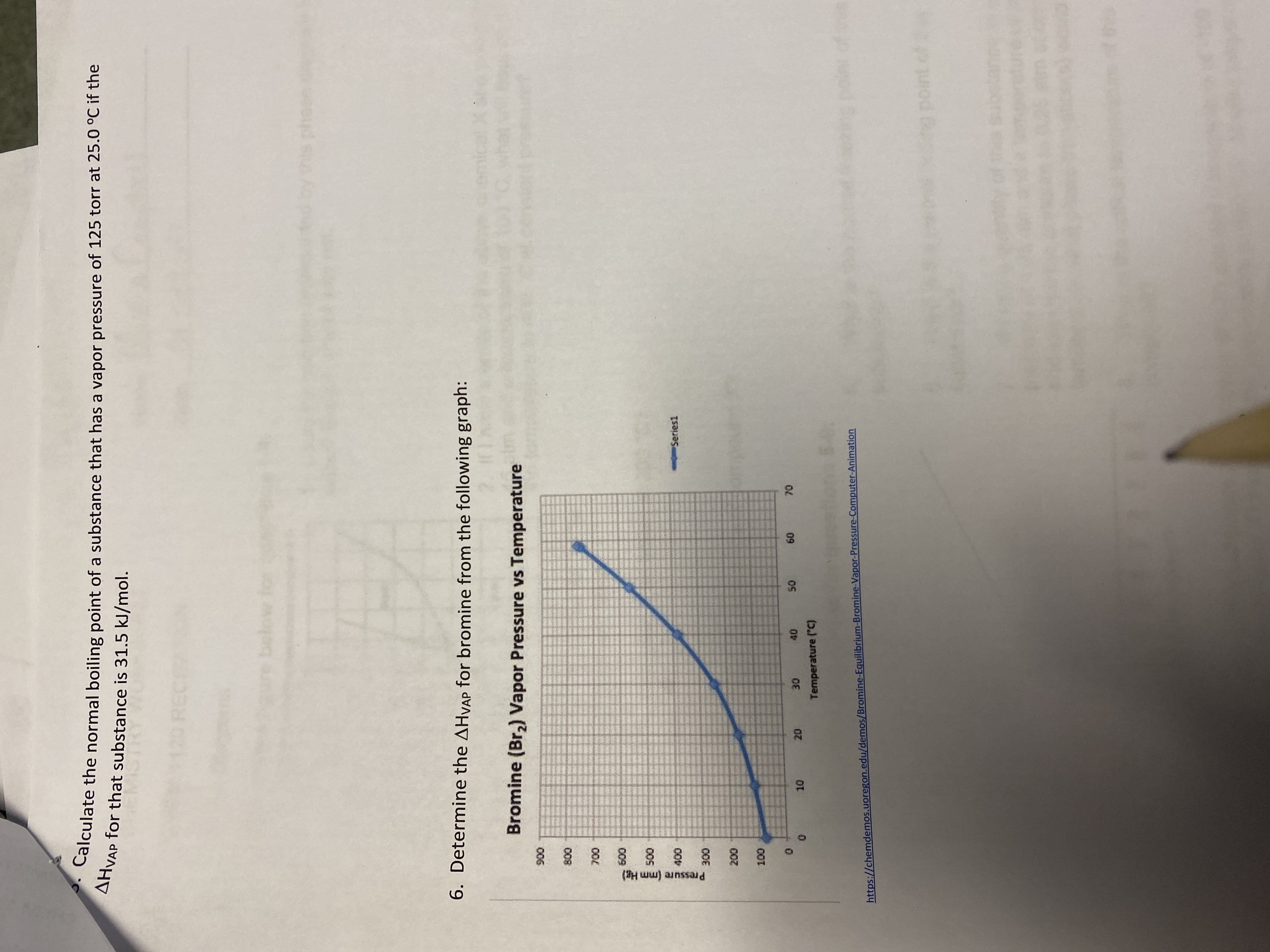
Transcribed Image Text:Caiculate the normal boiling point of a substance that has a vapor pressure of 125 torr at 25.0 °C if the
AHVAP for that substance is 31.5 kJ/mol.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The enthalpy of vaporization for methanol is 35.2 kJ/mol. Methanol has a vapor pressure of 101.3 kPa at 64.7 °C. Using the Clausius-Clapeyron equation, what is the vapor pressure for methanol at 44.9 °C? Give your answer in kPa, to the first decimal point.arrow_forwardkJ The enthalpy of vaporization of Substance X is 5.00 and its normal boiling point is -66. °C. Calculate the vapor mol pressure of X at -87. °C. Round your answer to 2 significant digits. || atm x10arrow_forwardplease explainarrow_forward
- A compound has a vapor pressure of 100 mmHg at 267 K and a normal boiling point of 338 K. What is the ΔHvap for this compound in kJ/mol?arrow_forward(a) Consider a substance where the intermolecular forces hold the molecules in fixed rigid positions. What is the process called when enough heat has been added to the substance so that the molecules begin to flow? (b) Consider a substance where the intermolecular forces hold the molecules in close contact with each other, but the molecules can flow. What is the process called when enough heat has been added to the substance so that the molecules escape each other? (c) Consider a substance where the intermolecular forces hold the molecules in fixed rigid positions. What is the process called when enough heat has been added to the substance so that the molecules can escape each other?arrow_forwardThe enthalpy of vaporization of a substance is 48.1 kJ/mol. The normal boiling point is 115oC. What is the vapor pressure of this substance at 63.7oC?arrow_forward
- 5.arrow_forwardThe normal boiling point of mercury (Hg) is 356.7 °C. What is the vapor pressure of mercury at 317.0 °C in atm? (AHvap = 58.51 kJ/mol)arrow_forwardThe enthalpy of vaporization for methanol is 35.2 kJ/mol. Methanol has a vapor pressure of 101.3 kPa at 64.7 °C. Using the Clausius-Clapeyron equation, what is the vapor pressure for methanol at 37.7 °C? Give your answer in kPa, to the first decimal point.arrow_forward
- Suppose the boiling point of pure water at high altitude is 85.00 °C. Use the Clausius-Clapeyron equation to determine the atmospheric pressure at this high altitude. The normal boiling point of water is 100.0 °C at 1 atm and its heat of vaporization is 40.7 kJ/mol.arrow_forward. The vapor pressure and enthalpy of vaporization of an unknown substance are measured to be 479.6 torr and 35.3 kJ/mol, respectively at room temperature (298.15 K). Estimate the normal boiling point of the substance at an atmospheric pressure of 760 torr.arrow_forwardA certain liquid has a vapor pressure of 92.0 Torr at 23.0 °C and 400.0 Torr at 45.0 °C. Calculate the value of AHvap for this liquid. AHvap = 52.3 Calculate the normal boiling point of this liquid. boiling point: 52 kJ/mol °Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY