
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Balance the chemical equation
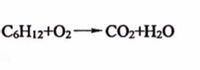
Transcribed Image Text:CH12+O2 CO2+H20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Balance chemical equation H2SO4+NaOH---->arrow_forwardBalance the equation: solid magnesium chloride and aqueous potassium phosphate react to produce aqueous potassium chloride and solid magnesium phosphate.arrow_forwardTranslate the following statements into chemical equations and then balance them. 1)Hydrogen gas combines with nitrogen to form ammonia 2)Hydrogen sulfide gas burns in air to give water and sulfur dioxide. 3)Barium chloride reacts with aluminum sulfate to give aluminum chloride and a precipitate of barium sulfate.arrow_forward
- In a chemical reaction, the substances present after the chemical change are called reactants. True Falsearrow_forwardWrite a balanced chemical equation based on the following description: the reaction of powdered aluminum and powdered iron(III) oxide produces solid aluminum oxide and liquid iron metalarrow_forwardWrite the word equation and then the chemical equation for the following reaction, And make sure it is balanced as well. When sodium metal reacts with iron(II) chloride, iron metal and sodium chloride are formed.arrow_forward
- WRITE THE WORD EQUATION AND CHEMICAL EQUATION REPRESENTING THE FOLLOWING REACTIONS. 1. SOLID POTASSIUM (K) AND CHLORIND (Cl₂2) GAS COMBINE TO FORM POTASSIUM CHLORIDE (KCI) POWDER.arrow_forwardWrite a balanced chemical equation based on the following description: liquid C₇H₈O is burned with oxygen gas to produce gaseous carbon dioxide and water vaporarrow_forwardBalance the following chemical equation NO(g)+o2(g)NO2(g)arrow_forward
- Balance the following chemical equation: C3H60 + 02 → CO2 + H20 O C3H60 + 402 → 3CO2 + 3H2O O C3H60 + 202 → 3CO2 + 3H20 O C3H60 + 402 → 3CO2+ 2H2O O 2C3H60 + 402→ 3CO2 + 3H2O O None of the above are balancedarrow_forwardBalance the following chemical reaction. Enter the sum of the balanced coefficients as your answer. Assign "blank" coefficients a value of 1. ammonia + oxygen gas -→ nitrogen monoride + waterarrow_forwardWrite the balanced chemical equation based on the following aqueous potassium phosphate reacts with aqueous nickel(ll) bromide to produce solid nickel (ll) phosphate and aqueous potassium bromidearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY