thank you can do part C Determine the fraction conversion occurring in the pond & the tank separately & determine the overall conversion of the process.
thank you can do part C Determine the fraction conversion occurring in the pond & the tank separately & determine the overall conversion of the process.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
thank you can do part C
- Determine the fraction conversion occurring in the pond & the tank separately & determine the overall conversion of the process.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
ok, i think im starting to get it, is it like this
can you please help me more with part C

Transcribed Image Text:C. Determine the fraction conversion occurring in the pond & the tank separately & determine the
overall conversion of the process.
NA entering pond = A
NA entering tank = B
NA leaving pond = C
NA leaving tank = D
X into pond
X into tank
=
=
A - B
A
A - D
A
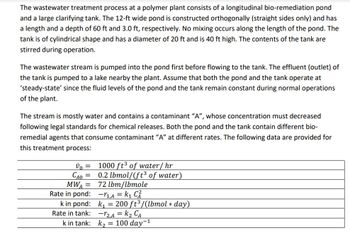
Transcribed Image Text:The wastewater treatment process at a polymer plant consists of a longitudinal bio-remediation pond
and a large clarifying tank. The 12-ft wide pond is constructed orthogonally (straight sides only) and has
a length and a depth of 60 ft and 3.0 ft, respectively. No mixing occurs along the length of the pond. The
tank is of cylindrical shape and has a diameter of 20 ft and is 40 ft high. The contents of the tank are
stirred during operation.
The wastewater stream is pumped into the pond first before flowing to the tank. The effluent (outlet) of
the tank is pumped to a lake nearby the plant. Assume that both the pond and the tank operate at
'steady-state' since the fluid levels of the pond and the tank remain constant during normal operations
of the plant.
The stream is mostly water and contains a contaminant "A", whose concentration must decreased
following legal standards for chemical releases. Both the pond and the tank contain different bio-
remedial agents that consume contaminant "A" at different rates. The following data are provided for
this treatment process:
vo=
CAO =
MWA =
Rate in pond:
k in pond:
Rate in tank:
k in tank:
1000 ft³ of water/ hr
0.2 lbmol/(ft3 of water)
72 lbm/lbmole
-₁,A = k₁ C²
k₁= 200 ft³/(lbmol * day)
-12,A = K₂ CA
k₂= 100 day-¹
Solution
Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The