
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Which oxygen atom has the greater electron density?
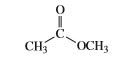
Transcribed Image Text:C.
CH
OCH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Potassium iodide (KI) exhibits predominantly ionic bonding. The K+ and I− ions have electron structures that are identical to which two inert gases?arrow_forwardChoose two atoms. List what their electron affinity values are including units. Which of these two would be more likely to form a bond with sodium metal? Why?arrow_forwardPlease draw energy level diagrams and electron configurations for the following: Tellurium ion Manganese (III) ion Arsenic ion Palladium (IV) ionarrow_forward
- The pattern that emerges for many atoms (and especially C, N, O, F) is sometimes referred to as the "octet rule." Hydrogen follows the same pattern, but there is no "octet" of electrons for hydrogen. Use electron configurations to explain why hydrogen is following the same pattern. Use the term "closed shell" in the explanation.arrow_forwardArrange the following ions in order of increasing ionic radius: potassium ion, calcium ion, sulfide ion, chloride ion. Smallest Largestarrow_forwardBriefly describe how to use the electron dot symbols of main group elements to determine the number of bonds they usually make.arrow_forward
- Explain why boron (B) has a higher ionization energy than fluorine (F).arrow_forwardDescribe the location of electrons; describe how electron placement determines chemical bonding, stability, and becoming an ionarrow_forward-Draw cesium, berryllium, bromine, and flourine by using both lewisand rutherford diagrams, and then write the size of each element.arrow_forward
- The Lewis structure for a boron atom has electron pair(s) (represented by :) and unpaired electrons (represented by -). Assume that electrons fill in the same order as an orbital diagram. 1; 1 1; 3 0; 3 2; 1 O 3: 0arrow_forwardWhat is the element has five 4p valence electrons? what ion will itform?arrow_forwardWrite electron configurations for the most stable ion formed by each of the elements , Ba, Se, and Cl (when in stable ionic compounds). (Express your answer as a series of orbitals. For example, the electron configuration of Li would be entered in complete form as 1s2 2s1 or in condensed form as [He]2s1.) a.) Electron configuration for the most stable ion of Ba b.) Electron configuration for the most stable ion of Se c.) Electron configuration for the most stable ion of Clarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY