
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
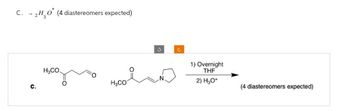
Transcribed Image Text:C. 2H₂O (4 diastereomers expected)
H3CO.
H3CO
J
0
1) Overnight
THF
2) H3O+
(4 diastereomers expected)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Compound: 4,5-dimethyl-4-cyclohexene-cis-1,2-dicarboxylic acid anhydride or C10H12O3 Label all IR peaksarrow_forwardCalculate the degree of unsaturation. Assign the relevant peaks in the IR spectrum. Assign the peaks in the 13C NMR spectrum. Assign the peaks in the 1H NMR spectrum.arrow_forwardUse the 1H NMR and IR spectra given below to identify the structure ofcompound B (molecular formula C4H8O2).arrow_forward
- Draw the structure of any diastereomer of (2 S S , 3 R R )-2,3-dichloropentane.arrow_forwardName the compoundarrow_forwardA student performed a series of reactions and obtained the spectral data provided. The reaction sequence is shown below.. What are the structures for A, B, C, and D? Compound B NMR (relative integrated areas below peaks) CH;CH,O°N 1. BHTHF N-bromosuccnimide A CH;CH,OH B 2. H,O,/OH- D hv reflux Compound A NMR (relative integrated areas below peaks) PPM 5 Compound B IR INFRARED SPECTRUM PPM 5 1 6 0.8 Compound A IR INFRARED SPECTRUM 0.6 0.8 0.4 0.4 0.2- 3000 2000 Wavenumber (cm-1) 1000 The MS of compound B shows an M+ peak at 198 and an M+2 peak at 200 with a relative abundance of 1:0.97 2000 Wavenumber (cm-1) 3000 1000 Transmitance Relative Transmittancearrow_forward
- If 25.5mg of triethylmethanol is produced when 0.100mL of methyl propanoate and 0.200g of ethyl magnesium bromide react, what is the % yield? CH3CH₂CO₂CH3 + Methyl propanoate 2 CH3CH₂MgBr + 2 H+ → (CH3CH₂)3COH + CH3OH + 2 MgBr* Ethyl magnesium bromide triethylmethanol methanolarrow_forwardIn the 1HNMR spectrum for 4-methylphenylboronic acid, assign the variousresonances to the hydrogen nuclei responsible for them.arrow_forwardplease help!!!! tyyyarrow_forward
- Name the following Cd(C2H3O2)4arrow_forward2. (Chapter 13 - Q58b) The compound whose H NMR spectrum is shown has the molecular formula C7H7B1. Follow the following questions to predict the unknown structure. Chem. shift Rel. area 2.31 1.50 701 1.00 7.35 1.00 TMS 10 7 6 O ppm 4 3 2 Chemical shift (8) e20s Cenge leaming 2(a) Degree of the unsaturation of this compound is= 2(b) The two distinct peaks in the aromatic region of the H NMR indicate that compound is .disubstituted = 2(c) The splitting pattern of the peak at 2.31 ő is = 2(d) The group that corresponds to the splitting pattern in 1(c) is = 2(e) This compound has the plane of symmetry Yes or No = 2(f) The name of the unknown compound = Intensityarrow_forward23Na has no NMR frequency. What is its magnetogyric ratio? A) 101.1 x 10\^7 T-1 s-1 B) 26.75 x 10\^7 T-1 s-1 C) 7.081 x 10\^7 T-1 s-1 D) 0.9377 x 10\^7 T-1 s-1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY