
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Firefly luciferin exhibits three rings. Identify which of the rings are aromatic. Identify which lone pairs are involved in establishing aromaticity. The lone pairs are labeled A-D below.
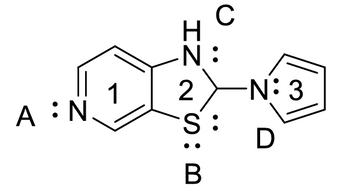
Transcribed Image Text:C
ΖΙ
-N:
1
2
·N: 3
A:
A N
S: B
S:
D
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 2.94 Use a molecular level description to distinguish between LDPE and HDPE.arrow_forwardAn element X bas five major isotopes, which are listed below along with their abundances. What is the element? Isotope Percent Natural Abundance Mass (u) 46x 8.00% 45.95232 47x 7.30% 46.951764 48x 73.80% 47.947947 49x 5.50% 48.947841 50x 5.40% 49.944792arrow_forward2.71 Use the web to determine the amount of low-density polyethylene and high-density polyethylene produced annually in the United States. Which uses predominate in the applications of these two materials?arrow_forward
- Name the following compounds: CsCl BaO K2S BeCl2 HBr AlF3 AlF3arrow_forwardClassify the following as compounds or elements: a silver bromide used in photography; b calcium carbonate limestone; c sodium hydroxide lye; d uranium; e tin; f titanium.arrow_forward2-100 A 0.100 g sample of magnesium, when combined with oxygen, yields 0.166 g of magnesium oxide. What masses of magnesium and oxygen must be combined to make exactly 2.00 g of magnesium oxide?arrow_forward
- Please don't provide handwritten solution .....arrow_forwardComplete the following chart by writing "LEFT", "RIGHT" OR "NONE" for equilibrium shift, and "INCREASES", "DECREASES" or "REMAINS THE SAME" for the concentrations of reactants and products when the indicated stressors are applied to the given system at equilibrium. 2 SO₂ (8) + O₂(g) ↔ 2 SO 3 (8) SISSA OOON NR E O > SON MA Equilibrium Shift Concentration of SO2 Concentration of 02 Concentration of SO3arrow_forwardA compound consists of elements combined in a fixed ratio. True or Falsearrow_forward
- INORGANIC CHEMISTRY MODULE ASSIGNMENT: Write the Formulas OF Compound in which combining ratios are as FollOWS Ca) sodium: Chlorine: ox7gen, 1:1:3 (b) Aluminnum Catomic symbol Al) : fluorine Catomic symbol F), 1:3arrow_forward77. Determine the number of protons and electrons in each ion. (a) Na+ (b) Ва?+ (c) O²- (d) Co³+arrow_forwardChemistryarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning