
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
If the group below was on a mono substituted benzene ring, would it be an ortho or para director?
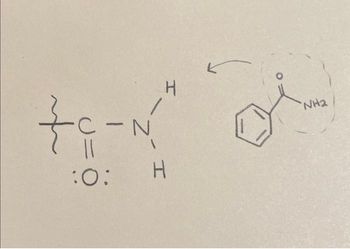
Transcribed Image Text:H
FC-N
H
::
/
'NH2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the following reaction in detailedarrow_forwardStyrene (shown below) has 8 n electrons but is aromatic. Why?arrow_forwardWhich one of the following statements from Chapter 8 and 18 is FALSE? O For a compound to be classified as aromatic, it must have an odd number of pairs of r-electrons and must be fully conjugated, cyclic and planar. O Inclusion of heteroatoms as part of the conjugated ring system renders the molecule to be considered non-aromatic. O Benzene is a six-membered ring where the r-electrons are equally shared across all six carbons in the ring and thus it possesses 36 kcal/mol of resonance stabilization energy. O All EAS reactions follow the same three mechanistic steps: (1) generate the electrophile, (2) capture the electrophile to give a carbocation intermediate, and (3) loss of a proton to regain aromaticity.arrow_forward
- For the structures shown below, state the number of pi electrons present in the molecule. ОН НО Number of pi electrons Number of pi electrons = || Is the molecule aromatic according to the Huckel criteria? If the molecule were planar, would it be antiaromatic?arrow_forwardBreak the molecule, 3-phenyl-prop-1-yne, such that you have separated the aromatic portion and the aliphatic portion. How much does each fragment weigh?arrow_forwardFill the empty boxes with compatible structure. (Give answer in clear handwritten)arrow_forward
- 12. Which of the following aromatic compounds (pyridine or pyrrole) is more basic? Explain. (Hint-think about the hybridization of each atom) IZarrow_forwardGive the curved arrow for the structure and predict the product H*arrow_forwardO Use the polygon rule to draw the MO energy diagram of the cyclononatetraenyl anion. Assuming planarity, would this ion be aromatic or antiaromatic?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY